Summary
In most cases, drugs are found to be very complicated in terms of their structural formulae which further complicate their reaction mechanisms. They are susceptible to both chemical and biological reactions. As they take p[art in these important molecular reactions, they eventually degrade and deteriorate further thereby losing their viability especially on the target organism. Both the chemical and biological reactions which lead to drug degradation usually commence immediately after the very drug has been manufactured or taken. Such types of decomposition processes often result into less effective drugs which cannot meet the intended purpose. If this happens, then the drugs are said to be low in efficacy. Additionally, decomposition of drugs might result into the formulation of poisonous substances thereby endangering the life of a patient. Hence, it is imperative to understand all the associated processes of decompositions that patients are not put under serious health risk. Some of the processes which can account for drug instability include hydrolysis, oxidation and reduction. As the process of any decomposition takes pace, it is only the functional groups in the structural formulae of drugs that take part in either the chemical or biological reactions (Yoshioka 210).
In this respect, there are mainly three processes which are associated with stability of drugs namely hydrolysis, oxidation and reduction. Hydrolysis refers to the process whereby an organic molecule reacts with water and both bonds in the organic substance and water are duly broken. However, reduction is the gain of electrons or the decrease in bonding level in an atom owing to the removal of oxygen.
Main Body
Functional groups which are theoretically susceptible to hydrolysis, oxidation and reduction
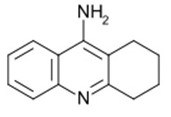
Tacrine
In Tacrine, the functional group susceptible for the degradation process is the amide (-NH2) group. For example, during the process of hydrolysis, the amide molecule interacts with water and eventually breaks.
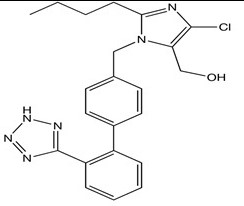
Losartan
Its functional group is the NH (part of the tetrazole ring) which is usually considered the reactive site of the drug since it is negatively polarized.
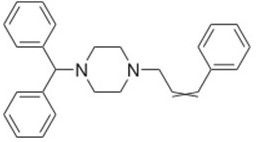
Cinnarizine
The functional group is the Nitride (N–) site which is negatively charged and hence can gain electrons during the process of decomposition.
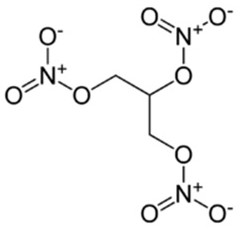
Nitroglycerin
The functional group is the NO3– .There are three similar functional groups in this drug which all react in a similar way during the process of decomposition.
Diltiazem
The carboxylic (COOH) functional group is the reactive site of this drug which undergoes degradation process
Evidence at molecular level why the identified functional groups are susceptible for degradation
Oxidation
Oxidation refers to the transfer of electrons whereby the target atom undergoes loss of electrons. In terms of the oxygen, it refers to a chemical process in which an atom increases the bonding level with oxygen or more of the latter atom is added to oxygen. For drugs to deteriorate through oxidation there must be presence diatomic oxygen (Connors et al. 212). The reaction can progress without any significant catalysis. Oxygen in its molecular state contains two radicals of electrons and which constitutes the electronic arrangement of oxygen when uncreative. Molecular oxygen has two atoms of oxygen covalently bonded to each other through equal sharing of electrons. Its structure may vary in relation to the theory under discussion. The unpaired electrons in an oxygen molecule are capable of initiating a chemical reaction on the target drug and this reaction may not reach completion over a short period of time especially if the reaction is under the influence of a catalyst.
Hydrolysis
The reaction between an organic compound and water constitutes hydrolysis reaction I which bonds are broken among the reacting species while there is formation of bonds as products are generated. It is similar to hydration which is basically the addition of water to a substance without the decomposition of the molecule (Cairns 217). Most drugs are susceptible to hydrolysis after synthesis. Nonetheless, the amide functional group is found to be more prone to this decomposition reaction. The amide functional group (NH2) undergoes a nucleophilic attack of the of the carbon atom which is part and parcel of the functional group called carbonyl. As a result, bond breaking between carbon and oxygen takes place thereby opening up the molecule. Another likely bond which can be cleaved is that of carbon and nitrogen because it is single hence requires minimal bond breaking energy. The oxygen atom is usually partially negatively charged making the carbonyl functional group to be positively polarized. This separation of charges within the organic molecule necessitates bond breaking through hydrolysis. It takes place rather slowly although the process can be hastened through acid or base catalysis.
Products of deterioration or decomposition of the drugs
The decomposition of an amide functional group leads to the formation of an alkanoic acid and alkylamoniun ion as shown in the equation below
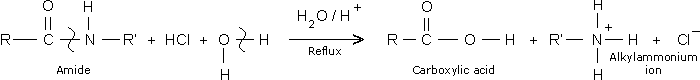
The deterioration of a carboxylic functional group (COOH) in the presence of a basic catalyst leads into the formation of neutral products namely water and salt and consequently rendering the drug ineffective. On the other hand, the NO3– functional group will decompose to other volatile oxides of nitrogen like nitrogen (I) oxide and the unstable nitrogen (II) oxide.
Precautions to be employed to minimize or prevent the degradation of the 5 drugs due to hydrolysis oxidation/reduction
There are quite a number of procedures which can be taken in order to alleviate the process of drug deterioration through oxidation. To begin with, the exclusion of oxygen from drug presence is the simplest and most direct step which can be taken to minimize the rate of oxidation (Cairns 211). This procedure can be accomplished through using an alternative gas to oxygen and which must be inert or generally non reactive under ordinary conditions. Rare gases such as argon or neon can be used in this case. Similarly, nitrogen has been found to be relatively inert and can substitute oxygen should need be. The drug is placed in an airtight container to prevent air oxygen from getting into it.
Colored Glass containers
An ambered glass container is capable of dispelling light energy of certain wavelengths. For this reason, drugs which are chemically hypersensitive to light can be protected through the use of such containers. Light energy is kept at bay and hence the drugs cannot be decomposed within a limited period of time (Cairns 212).
Chelating agents
Small metallic ions like those from transitional elements are capable of acting as catalysts in the process of oxidation. This poses an even greater threat to the process of decomposition of drugs especially if the latter is stored in such reactive containers. As a precaution, it is advisable to store these drugs in non metallic containers like glass or preferably stainless steel. Nevertheless, chelating agents like EDTA can be made use of in cases where it is inevitable to avoid contact with metallic ions (Huynh-Ba 79). These agents have the ability of eliminating any metal ions present and hence reducing the rate of oxidation.
Antioxidants
Alternatively, the use of antioxidants can be effective in minimizing the rate of drug deterioration. These substances have the ability of oxidising themselves rapidly and hence reducing the likelihood of the target drugs undergoing the same within a specified container. The free radicals which are formed after antioxidants have been oxidized cannot adequately permit the continuation of oxidation. A good example of an antioxidant is ascorbic acid (Carstensen &Rhodes 67).
Conclusion
In summing up this paper, it is imperative to recap a few details which are worth noting. To begin with, drug stability is found to deteriorate depending on the duration and intensity of both physical and chemical conditions. As noted earlier, oxidation plays a very instrumental role in the decomposition process of the drugs mentioned. This can take place either as electrons are being lost from the target atom or oxygen is being gained by the respective atom thereby increasing the number of bonds. Additionally, the functional group of an organic molecule as in the case of the drugs studied above refers to the reactive site of the molecule an d is usually polarized due to the separation of charges. It is these functional groups that are susceptible to degradation as the drug decomposes. From this decomposition process, bonds are broken and new products are formed. This may not be beneficial to the effectiveness of the synthesized drugs due to low efficacy as the drug decomposes.
Works Cited
Anon. Hydrolysis of Organic Compounds. N.d. Web.
Cairns Donald. Essentials of Pharmaceutical Chemistry (3rd ed).London: Pharmaceutical Press, 2008. Print.
Carstensen Thuro Jens and Rhodes T. Christopher. Drug Stability: Principles and Practices, New York: Marcel Dekker Inc., 2000. Print.
Connors A. Kenneth et al. Chemical stability of pharmaceuticals: a handbook for pharmacists, New Jersey: John Wiley and Sons Inc., 1986. Print.
Huynh-Ba Kim. Pharmaceutical Stability Testing to Support Global Markets, New York: Springer, 2010. Print.
Yoshioka Sumie and Stella J. Valentino. Stability of drugs and dosage forms. New York: Kluwer Academic publishers, 2000. Print.