Industry: Scope and Boundaries
The current focus of the medical device production industry seems to be on developing smart and connected products. The reason for the identified change concerns the need to create an efficient information management framework. Thus, patients and healthcare exports remain well informed, which allows them to make the best decision when addressing a particular problem (Narula, 2016).
Therefore, when considering the inside issues of the industry, one must mention the propensity to focus on using the latest technologies. The importance of communication enhancement tools should be mentioned among the strongest tendencies. When evaluating the impact of the outside forces, one should consider the demographic factor as driving one behind the industry’s development.
Fisher and Paykel and Porter’s Five Forces Model
Porter’s Five Forces Analysis, Fisher and Paykel, which is one of the most influential organizations in the industry, can be deemed as fairly successful. Its prospects in the target market are currently rather uplifting. With a heavy emphasis on R&D and impressive investments, Fisher and Paykel have a huge potential in the medical device industry.
Table 1. Porter’s Five Forces Analysis: Fisher and Paykel
However, as the analysis results show, there is also a threat of the current competitors taking over the industry and ousting Fisher and Paykel from the market. Therefore, the organization must be able to produce innovative solutions and improve product quality. Thus, Fisher and Paykel will retain their competitive advantage.
Making High Returns and Mitigating Unfavorable Forces
In order to address the threats of the global market, Fisher and Paykel will have to focus on building a strong competitive advantage. For this purpose, investing in R&D and quality management should be viewed as a necessity. It can be suggested that the principles of Six Sigma and especially the DMAIC Model, should be incorporated into the firm’s processes so that the course for consistent quality improvement could be set.
Competitive Forces: Possible Evolution Course
Succeeding in the medical device industry is a complicated yet possible task for an organization that focuses on R&D and quality management. At present, several major companies dominate the industry, whereas smaller ones take minor niches. The fact that only a few firms are currently at the helm of the medical device market makes the identified industry a highly competitive environment.
Niche products and innovative solutions can be viewed as the primary competitive drivers behind the development of the industry (Pyzdek & Keller, 2014). Therefore, it is recommended that Fisher and Paykel should focus on the enhancement of the R&D processes. Particularly, the introduction of IT innovations into the company’s processes, as well as the end products, should be deemed as the course for the further development of the organization. More efficient use of the hospital space and the focus on home care should also be considered as possible areas of development.
Strategic Groups Analysis
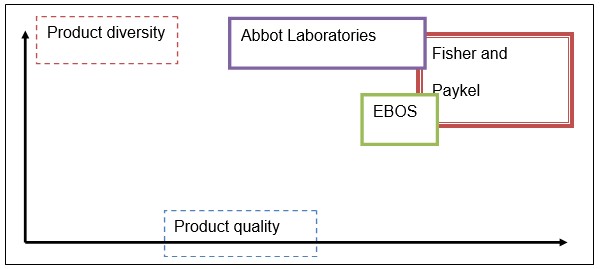
As the chart above shows, Fisher and Paykel are currently delivering diverse products of the finest quality. However, the identified tendency may change in the future unless the course for unceasing improvement and innovation is set. Thus, R&D and quality management should be currently viewed as the areas that deserve the greatest attention and the largest number of investments.
References
Narula, R. (2016). The modern MNE as an efficient meta-integrator: Emerging market mines need to foster internal embeddedness to succeed. Henley-on-Thames, UK: Henley Business School.
Pyzdek, T., & Keller, P. (2014). The Six Sigma Handbook (4th ed.). New York, NY: McGraw-Hill Education.