Introduction
Flash chromatography is a useful technique for faster purification of reactions products in synthetic organic chemistry common in pharmaceutical industry. It is used when the resolution required is low and the required capacity is high. “Packed columns, containing ca. 100g of irregularly shaped silica gel particles are eluted with organic solvents by gravity flow. To speed up the process, gas pressure (up to 100 psi) can be applied to the solvent reservoir (Heftmann 512).”
This method was popularized as an alternative to the gravity- fed chromatography which is infamous for being slow and very inefficient, by Clark Still from Columbia University.
Flash chromatography column
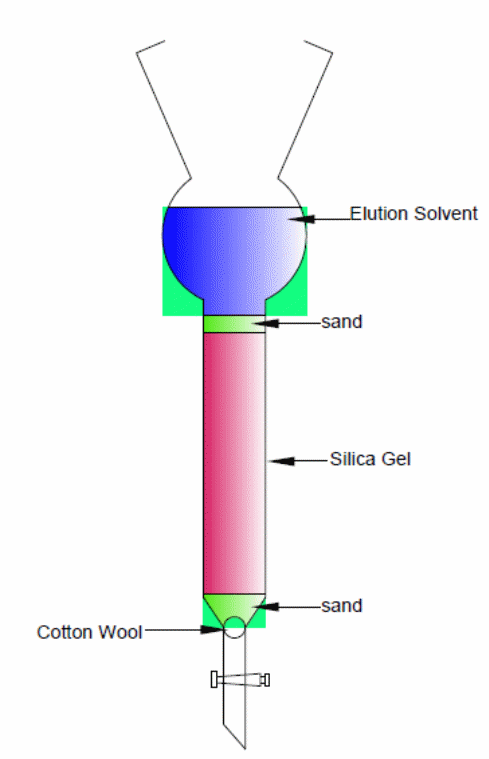
Procedure
Select a solvent system: The solvent system selected should be such that one solvent has a higher polarity than the other and the compound of interest have a TLC RF of approximately 0.15 to 0.20. It should be noted that, the polarity of the system and thus the elution rates are dependent on the solvents ratios. Hence, the rate of elution increases with rise in polarity.
Determine the silica gel quantity: Silica gel quantity will be dependent on the sample amount and the compounds RF difference. For example, if you have k grams of a sample, then you will need approximately 30 to 100k grams of silica gel. It is important to note that, when more of silica gel is used, the time required for the chromatography will be longer. Hence, for faster separation, ratios of 30:1 or closer would be more effective.
Packing the column: Use a glass column with a cotton wool plug above the stopcock. The cotton wool is important in preventing the silica gel’s escape through the stopcock. Then place a clean layer of sand above the plug till a flat surface is obtained whose diameter equals the column’s body, then pour the silica gel. Since silica is carcinogenic, the above operation should strictly be carried out in the hood.
Solvating silica gel column: Ensure the silica gel has settled by gently tapping the column sides. Pour the elution solvent to the silica gel then, using a gas pump, force the solvent through the silica. You will notice that as the pump forces more of the solvent, the silica gel becomes more and more homogenous in appearance because the entrapped air is forced out. Therefore, the pump should run till all the silica gel becomes homogeneous.
Apply the sample: Any solvent remaining on top of the silica should then be allowed to drain till it is flush with the silica’s top surface, which must be flat. Without disturbing silica’s top surface, the dissolved sample in the elution solvent is then gently applied and allowed to soak. Using very small amounts of the elution solvent, the sides of column is then rinsed and allowed to soak into silica gel before a layer of sand is added to the silica gel’s top surface as a protection when more of the solvent is added.
Eluting the sample: Add the solvent to the column, and apply a minimum pressure enough to force the solvent through the column and out, in a steady stream. Discard the first samples of the solvent coming out; an amount equal to the dry silica’s volume used, because it will take some time before the expected compound begins to elute, and then collect the remaining eluted solvent into test tubes.
Locate the sample: locate the fractions in your compound and analyze them using TLC, then using rotary evaporator, concentrate the combined fractions.
Clean the column: clean the column using a gas pump and remove any remnants using a vacuum from the aspirator, applied from the column’s bottom.
Reference
Heftmann, E. “Chromatography 6th Edition.” Fundamentals and applications of chromatography and related differential migration methods. Part A: Fundamentals and Techniques 69A (2004): 512