Case Analysis
Helsmoortel-Van der Aa syndrome in a 13-year-old girl with autistic spectrum disorder, dysmorphism, right solitary kidney, and polycystic ovaries
Abstract
Helsmoortel-Van der Aa syndrome (HVDAS) is a disease officially documented in 2014. It is characterized by neurodegenerative disorders, reduced intellectual and motor function, facial dysmorphism, and an autism spectrum disorder. Many patients are also characterized by neuropsychiatric disorders, including attention-deficit/hyperactivity disorder, anxiety disorders, and various behavioral abnormalities. We report a 13-year-old patient with autosomal dominant Gelsmortel-Van der Aa syndrome with a probable pathogenic variant of the ADNP gene de novo. The patient had a right-sided solitary kidney and polycystic ovaries conditions not previously associated with this syndrome.
Keywords: Helsmoortel-Van der Aa syndrome; neurodevelopmental disorder; ADNP gene; intellectual impairment; facial dysmorphism.
Summary
Helsmoortel-Van der Aa syndrome (HVDAS) is a relatively recently identified disorder of neurogenic nature, which was documented in 2014 in 10 people. It was identified by a group of scientists led by Helsmoortel et al., who found that the disease occurs in response to autosomal dominant de novo ADNP mutations (MIM: 615873) (Helsmoortel et al., 2014). Intellectual and developmental delays in the child characterize the disease. In addition, facial dysmorphic features and sometimes congenital heart defects occur (Helsmoortel et al., 2014). The frequency of this condition as part of the autism spectrum caused by genetic causes does not exceed 1% of autism cases.
The literature review analyzed the diagnostic criteria used to define HVDAS. Pascolini et al. (2018) report the need for molecular analysis of the ADNP gene when ASD traits are observed in conjunction with facial dysmorphism: a forehead with a high anterior hairline, blepharophimosis, palpebral ptosis, and hypertelorism. The molecular basis of this syndrome is evidenced by the haploinsufficiency of the ADNP gene (OMIM #611386). When this gene is deficient, there can be disorders of transcription (Pascolini et al., 2018) and p53 cell apoptosis mechanisms (Bassan et al., 1999). The same results are also reported by Zamostiano et al. (2001) and Magen et al. (2014), who indicate that apoptosis is induced when antisense oligonucleotides silence ADNP production. ADNP deficiency in mice is characterized by an increase in transcripts of genes associated with lipid metabolism (Mandel et al., 2007). The complete absence of the gene leads to the death of the pregnant mouse on day 9 (Pinhasov et al., 2003). Such data point to expanding the presentation of clinical cases associated with severe HVDAS syndrome.
The present study analyzes a clinical case: a 13-year-old girl with an ADNP mutation and characteristic neuropsychiatric features of the disease.
Case Report
An expert clinical geneticist performed a clinical evaluation. Written informed consent for inclusion in the study and publication of photographs was obtained from the parents. A 13-year- old girl was referred at the age of 10 years to the clinical genetic and metabolic disorder clinic in King Abdullah Specialist Children Hospital–Riyadh after presenting with dysmorphic features and severe mental retardation, ASD, and attention deficit/hyperactivity disorder, according to the mental health professional clinic evaluation. She complained of sleep disturbance and frequent attacks of apnea. The patient was the child of a first-degree cousin Saudi parents and was born at term by an emergency cesarean section after a pregnancy complicated by preeclampsia. She had no significant perinatal history. The patient’s early development was normal; she started sitting without support at seven months and began walking at 12 months; however, she exhibited speech delay, starting at the age of
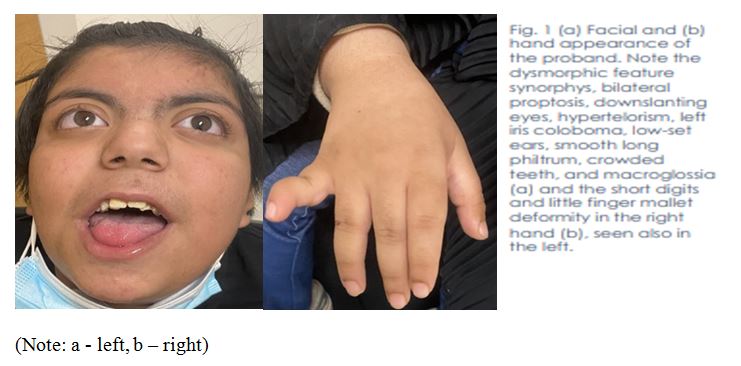
At our visit, her weight was 80 kg (>97th percentile), and she was morbidly obese according to the Saudi population growth charts. Her height and head circumference were within normal ranges. Phenotypical examination revealed hirsutism, bilateral proptosis, down-slanting eyes, hypertelorism, left iris coloboma, low-set ears, smooth long philtrum, crowded teeth, and macroglossia. She had short digits and a slight finger mallet deformity bilaterally in her hands (Fig. 1), joint laxity with recurrent ankle strain accidents, episodes of left calcaneus, and distal fibula osteomyelitis.
Solitary right kidney and bilateral ovarian cysts were found in abdominal radiological investigations. Brain magnetic resonance imaging showed hypomyelination in the peritrigonal areas but was otherwise unremarkable. A cardiac echocardiogram revealed trivial aortic, tricuspid, and mitral valve insufficiency and closure by a device secondium atrial septal defect. A Holter monitor connected for 24 h revealed a predominant sinus rhythm and first-degree atrioventricular block. Basic biochemistry and metabolic workup were unremarkable.
After obtaining informed consent, we performed genetic studies. The results of conventional chromosome analysis and CGH-array of the peripheral lymphocytes were average. We performed clinical exome analysis to determine the genetic basis of the condition, which showed a heterozygous likely pathogenic variant in ADNP gene c.1265dup p. (Gln423Serf*17), creating a shift in the reading frame starting at codon 423. The new reading frame ends in a stop codon 16 positioned downstream, which is consistent with the genetic diagnosis of autosomal-dominant HVDAS. Clinical exome analysis confirmed the presence of the mutation in the proband and absence in her parents, supporting its de novo nature.
Discussion
Autosomal dominant de novo mutations in ADNP (MIM: 615873) are an effective mechanism of HVDAS syndrome development. About 0.17% of mutations are associated with the development of ASD, so it can be considered one of the red flags of autism development (Helsmoortel et al., 2014). Extensive sequencing capabilities allow us to identify and understand the causes of neurodevelopmental disorders more accurately, assembling the basis for a genetic library. Autism spectrum disorders are common and affect about one in 59 children (Shillington et al., 2020). High variation in the ADNP homeobox gene can lead to the development of syndromic autism, exacerbating the behavioral problem in about 78% of cases. The treatment efficacy of ASD cases mediated by disorders of the ANDP gene is relatively high with Risperedine (Shillington et al., 2020). However, pharmacological therapy remains limited because the effects of drugs in combination with genetic disorders are not fully understood.
Patients with ADNP disorder are complex clinical cases because gene transcription disorders do not always mediate the observed manifestations. There are likely other underlying problem-induced abnormalities that result in mental and motor delays, as well as abnormalities of body and facial structure (Pascolini et al., 2018). Global developmental delay, difficulty eating, and regular acts of cyanosis are additional signs of HVDAS (Pescosolido et al., 2014). In addition, mood disorders combined with attention deficit/hyperactivity disorder appear to be direct consequences of children’s mental and motor retardation at an early age.
It should be noted that variation in the manifestation of de novo mutations in the ADNP gene is not a mandatory predictor of neuropsychological and physiological disorders. The Deciphering Developmental Disorders Study (2015) reported clinical findings in four cases that had similar features. Specifically, they all had moderate general mental retardation, and almost all were accompanied by visual limb abnormalities and abnormalities related to primary and general metabolism. Two patients had plagiocephaly as well as hair disorders (Deciphering Developmental Disorders Study, 2015). In the worldwide cohort of patients with HVDAS, there are 78 patients under 40 years of age, 73 of whom are determined to have mental retardation (Van Dijck, et al., 2019). On average, delayed speech and motor development are seen in 70.5 individuals, conduct disorder in 48 individuals, and hypotonic conditions in 54 patients. In addition, eating disorders and gastrointestinal problems were present in the majority of patients, with 60 individuals (Van Dijck, et al., 2019). Such data indicate that despite the variability of clinical manifestations, they can be assigned to several groups and associated with specific disorders of human body departments.
The development of ADNP-associated syndrome can be observed at a very early age. Shillington et al. (2020) reports the discovery of genetic-associated ASD, which was suspected after examination of a congenital diaphragmatic hernia. The patient had global developmental delay, early tooth formation, and vision problems. In addition, the patient was unresponsive to behavioral treatment, and the congenital heart defect aggravated feeding, breathing, and motor activity. Genomic changes in DNA methylation have been reported in 22 people with HVDAS, allowing the syndrome to be classified as a systemic epigenetic disorder (Breen et al., 2020). This is likely to allow the introduction of methylation tools or associated therapies to achieve results in behavioral function modification. Abnormalities in DNA methylation were also confirmed in study by Bend et al. (2019) , where the authors systematized the available features and made a classification. They found two different and partially opposite episignatures of genomic DNA methylation in 22 patients. The epi-ADNP-1 episignature included ~6,000 predominantly hypomethylated CpGs, and the epi-ADNP-2 episignature included ~1,000 predominantly hypermethylated CpGs (Bend et al., 2019). The two obtained signatures correlated with the location of ADNP mutations.
Unusual manifestations of ADNP abnormalities include dental lesions, demineralization of enamel, and detection of dark stains on the enamel. This has been documented in the 9-year-old boy’s case who also had lower oral mucoceles (Petruzzi et al., 2021). However, there is currently no proven mediated relationship between dental lesions in ADNP syndrome, so it is impossible to judge the clinic from a single case fully.
Our patient demonstrated the molecular and clinical phenotype of HVDAS but also had a right solitary kidney and polycystic ovaries, which are not mentioned in the literature. Congenital heart defects in this disease are less common; our patient had significant congenital heart anomalies, with a second-order atrial septal defect requiring surgery and device closure not previously reported. The putative causative genetic variant is a de novo frameshift mutation c.1265dup p. (Gln423Serf*17), creating a frameshift starting at codon 423. The new reading frame ends with stop codon 16 located downstream; this variant has not been previously reported in HVDAS cases. Renal abnormalities and polycystic ovarian disease may be associated with this genetic subtype of HVDAS or with HVDAS in general.
References
Bassan, M., Zamostiano, R., Davidson, A., Pinhasov, A., Giladi, E., Perl, O., Bassan, H., Blat, C., Gibney, G., Glazner, G., Brenneman, D. E., & Gozes, I. (1999). Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. Journal of Neurochemistry, 72(3), 1283–1293. Web.
Bend, E. G., Aref-Eshghi, E., Everman, D. B., Rogers, R. C., Cathey, S. S., Prijoles, E. J., Lyons, M. J., Davis, H., Clarkson, K., Gripp, K. W., Li, D., Bhoj, E., Zackai, E., Mark, P., Hakonarson, H., Demmer, L. A., Levy, M. A., Kerkhof, J., Stuart, A., Rodenhiser, D., … Sadikovic, B. (2019). Gene domain-specific DNA methylation episignatures highlight distinct molecular entities of ADNP syndrome. Clinical Epigenetics, 11(64). Web.
Breen, M. S., Garg, P., Tang, L., Mendonca, D., Levy, T., Barbosa, M., Arnett, A. B., Kurtz-Nelson, E., Agolini, E., Battaglia, A., Chiocchetti, A. G., Freitag, C. M., Garcia-Alcon, A., Grammatico, P., Hertz-Picciotto, I., Ludena-Rodriguez, Y., Moreno, C., Novelli, A., Parellada, M., Pascolini, G., … De Rubeis, S. (2020). Episignatures Stratifying Helsmoortel-Van Der Aa Syndrome Show Modest Correlation with Phenotype. American Journal of Human Genetics, 107(3), 555–563. Web.
Deciphering Developmental Disorders Study. (2015). Large-scale discovery of novel genetic causes of developmental disorders. Nature, 519(7542), 223–228. Web.
Helsmoortel, C., Vulto-van Silfhout, A. T., Coe, B. P., Vandeweyer, G., Rooms, L., van den Ende, J., Schuurs-Hoeijmakers, J. H., Marcelis, C. L., Willemsen, M. H., Vissers, L. E., Yntema, H. G., Bakshi, M., Wilson, M., Witherspoon, K. T., Malmgren, H., Nordgren, A., Annerén, G., Fichera, M., Bosco, P., Romano, C., … Van der Aa, N. (2014). A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nature Genetics, 46(4), 380–384. Web.
Magen, I., & Gozes, I. (2014). Davunetide: Peptide therapeutic in neurological disorders. Current Medicinal Chemistry, 21(23), 2591–2598. Web.
Mandel, S., Rechavi, G., & Gozes, I. (2007). Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Developmental Biology, 303(2), 814–824. Web.
Pascolini, G., Agolini, E., Majore, S., Novelli, A., Grammatico, P., & Digilio, M. C. (2018). Helsmoortel-Van der Aa Syndrome as emerging clinical diagnosis in intellectually disabled children with autistic traits and ocular involvement. European Journal of Paediatric Neurology : EJPN : Official Journal of the European Paediatric Neurology Society, 22(3), 552–557. Web.
Pescosolido, M. F., Schwede, M., Johnson Harrison, A., Schmidt, M., Gamsiz, E. D., Chen, W. S., Donahue, J. P., Shur, N., Jerskey, B. A., Phornphutkul, C., & Morrow, E. M. (2014). Expansion of the clinical phenotype associated with mutations in activity-dependent neuroprotective protein. Journal of Medical Genetics, 51(9), 587–589. Web.
Petruzzi, M., Stella, A., Capra, V., Contaldo, M., & Della Vella, F. (2021). Oro-Dental Manifestations in a Pediatric Patient Affected by Helsmoortel-Van der Aa Syndrome. International Journal of Environmental Research and Public Health, 18(17), 8957. Web.
Pinhasov, A., Mandel, S., Torchinsky, A., Giladi, E., Pittel, Z., Goldsweig, A. M., Servoss, S. J., Brenneman, D. E., & Gozes, I. (2003). Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain research. Developmental Brain Research, 144(1), 83–90. Web.
Shillington, A., Pedapati, E., Hopkin, R., & Suhrie, K. (2020). Early behavioral and developmental interventions in ADNP-syndrome: A case report of SWI/SNF-related neurodevelopmental syndrome. Molecular Genetics & Genomic Medicine, 8(6), e1230. Web.
Van Dijck, A., Vulto-van Silfhout, A. T., Cappuyns, E., van der Werf, I. M., Mancini, G. M., Tzschach, A., Bernier, R., Gozes, I., Eichler, E. E., Romano, C., Lindstrand, A., Nordgren, A., ADNP Consortium, Kvarnung, M., Kleefstra, T., de Vries, B. B. A., Küry, S., Rosenfeld, J. A., Meuwissen, M. E., Vandeweyer, G., … Kooy, R. F. (2019). Clinical presentation of a complex neurodevelopmental disorder caused by mutations in ADNP. Biological Psychiatry, 85(4), 287–297. Web.
Zamostiano, R., Pinhasov, A., Gelber, E., Steingart, R. A., Seroussi, E., Giladi, E., Bassan, M., Wollman, Y., Eyre, H. J., Mulley, J. C., Brenneman, D. E., & Gozes, I. (2001). Cloning and characterization of the human activity-dependent neuroprotective protein. The Journal of Biological Chemistry, 276(1), 708–714. Web.