Introduction
Purpose of Experiment
The main purpose of the study is the research of morphological and cultural features of Escherichia coli and Staphylococcus aureus through Gram staining. Additionally, the objectives include the practice of the method and the accumulation of empirical material for the verification of findings from the literature review.
Gram Stain: Method Review
The method was developed by Christian Gram in 1884 (Tortora, Funke, & Case, 2015). Gram stain allows the categorization of bacteria into two groups based on the color of the cells after staining. After the procedure, it is easy to see the form, structure, and size of cells.
Escherichia Coli
The regular size of Escherichia cells is 2-6 microns in length and 0.4-0.6 mm in width. The bacteria have flagella enabling them to move. When plated in a solid medium, E. coli germination has either S- or R-form. When planted in a liquid medium, Escherichia shows sediment and turbidity (VanMeter, VanMeter, & Hubert, 2015). The shell of E. coli consists of polysaccharide and polypeptide layers. It has a small amount of peptidoglycan without cross-linking. Over the murein layer, there is the periplasmic space that plays an important role in the metabolic processes of the cell. The outer membrane contains lipopolysaccharide consisting of three components: A lipid, the core, and the polysaccharide chain formed in repeated oligosaccharide sequences (VanMeter et al., 2015).
Staphylococcus Aureus
The Staphylococcal cells have a spherical shape with an average diameter of 0.8-0.9 microns. In an axenic culture, staphylococci are represented in clusters, but it is also possible to find single or paired cocci in the analyzed purulent content. The Gram-positive cocci do not form capsules and spores; they are immobile (Mohamad, Jusoh, Htike, & Win, 2014). Gram-positive bacteria have a thick, durable, and simply arranged wall (50 nm thick). The main component of the bacteria’s cell wall is multilayered peptidoglycan (murein) built using N-acetyl muramic acid. Cross-linking strengthens the linear polymer of the cell. The teichoic or lipoteichoic acids are linked with peptidoglycan. Teichoic acid molecules are adhesion receptors (adhesins) (VanMeter et al., 2015).
Materials and Methods
I started the experiment by cleaning and labeling the slides for the smears. Then I placed a small drop of water on the first slide and stirred a small sample of E. coli culture into it. I carefully mixed the smear to ensure the bacteria is well spread. I used the heat fixing technique to adhere to bacteria to the slide. I proceeded to Gram staining: flooded it with crystal violet, washed it with the cleansed water after 30 seconds of waiting, added iodine for another 30 seconds, washed it with the alcohol-based bleach for 15 seconds, and added red dye for 30 seconds. Finally, I washed the smear with the water and waited until it dried. Afterward, I continued with the microscope examination.
All steps were repeated for other types of microorganisms.
Results
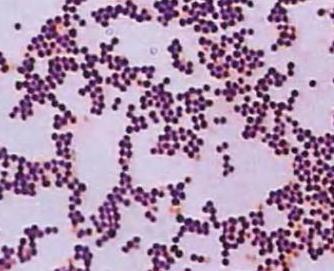
Methods
The purple color was obtained in three steps: coloring with crystal violet, fixation, and decolorization.
Description
The cocci appeared in the grape-like clusters. Each bacterium had a circular shape, moderate size, convex elevation. The microorganisms had an opaque optical property.
Results
At the end of the experiment, after bleaching, the bacteria remained purple which means that the main coloring agent bound with their murein layer extensively. The results are consistent with the theoretical data showing that S. aureus belongs to the category of Gram-positive bacteria.
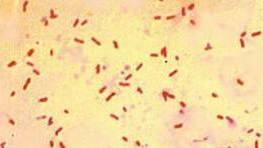
Methods
Along with coloring, fixation, and decolorization, the contrasting red colorant agent was applied to make the microorganisms distinct.
Description
Most of the bacilli had a circular, prolonged shape. However, some of the cells had the shape of coccobacillus – oval and short. All bacteria were located in a chaotic order and were small in size. They had slightly raised elevation and a smooth texture. The optical property of the microorganisms was translucent.
Results
After washing with alcohol and adding the red dye, the bacteria became pink. The findings indicate that E. coli belongs to the category of Gram-negative bacteria and, in this way, they conform with the theoretical data.
Discussion
When the process of staining was over, the smears adopted color associated with the microorganisms of their category. The microscopic analysis also allowed taking a close examination of the morphological features of the bacteria. The findings were similar to the information provided in the literature. S. aureus had a round shape, while E. coli had a prolonged form. Since E. coli culture was planted in the liquid medium, a high level of turbidity was observed. At the same time, S. aureus clusters had a complex, tetrad-like form.
The fact that the results were similar to the evidence provided in the literature indicates that the experimental procedure was carried out correctly. However, for the next analysis events, it is possible to extend the time of coloring with crystal violet and iodine solution to 1-2 minutes to obtain clearer pictures. Nevertheless, the results of the current experiment may be considered positive.
Conclusion
The experiment verified that E. coli is Gram-negative while S. aureus is the genus of Gram-positive bacteria. In the conducted Gram stain analysis, the main coloring agent actively bound with the murein layer. Since this layer is very thin in all Gram-negative bacteria and is screened by the outer membrane, E. coli microorganisms absorbed less dye than Gram-positive microorganisms. In the case of S. aureus, in the reaction with iodine, the dye and the cellular murein formed a complex which was hard to wash away with the bleaching solutions. Since all Gram-positive bacteria have a thick murein layer, the differentiation solutions could not completely wash the dye out of them.
Based on the observations mentioned above, it is possible to say that the most important conclusion is that the colors obtained during the process helped to correlate the empirical observations with the data regarding the tinctorial properties of the evaluated microorganisms, i.e., the genetic/biological content of the cells and their reactions with the chemicals. It means that the bacteria have different structures, and their shells largely affect the level of their resistance to various environmental influences.
References
- Mohamad NA, Jusoh NA, Htike ZZ, Win SL. 2014. Bacteria identification from microscopic morphology using Naïve Bayes. International Journal of Computer Science, Engineering and Information Technology 4(2): 1-9. Web.
- Tortora GJ, Funke BR, Case, CL. 2015. Microbiology: an introduction. San Francisco, CA: Pearson Benjamin Cummings.
- VanMeter K, VanMeter WG, Hubert, RJ. 2015. Microbiology for the healthcare professional. Maryland Heights, MO: Elsevier Health Sciences.