Introduction
The presence of liquid water sustains life on the planet. Liquid water provides an environment that allows for the easy dissolution and transportation of key elements and nutrients. A closer look at the digestive system, excretory system, photosynthesis, Krebs Cycle, and chemical reactions within an organic body requires the presence of a stable substance like liquid water. It is possible to have these properties due to the presence of hydrogen bonds within the molecules of liquid water. It is the presence of hydrogen bonds that allows liquid water to sustain and regulate life on planet Earth.
Hydrogen Bonding
Before going any further, it is important to describe the chemical properties and the molecular framework associated with hydrogen bonds within water molecules (Acta Crystallographica, 2017). A deeper understanding requires knowing the chemical properties of a water molecule. It is important to realize the covalent bond in a water molecule when a hydrogen atom bonds to the electronegative oxygen atom (Watson, 2017).
The interaction between hydrogen and oxygen atoms created a strong polar covalent bond (Watson, 2017). At the same time, this action creates a decentralization effect that causes the hydrogen atom to acquire a slightly positive charge (Watson, 2017). In the end, the hydrogen atom creates an electrostatic attraction with a negatively charged oxygen atom (OMICS International, 2014). The said electrostatic force rooted in the positively charged hydrogen atom is known in the scientific community as the hydrogen bond (OMICS International, 2014).
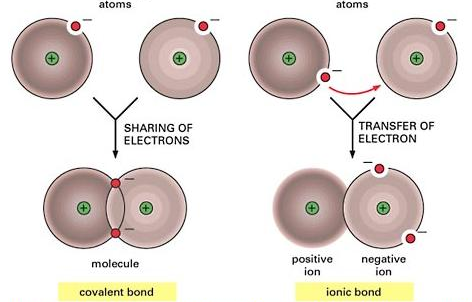
It is important to determine the strength of the hydrogen bond because this information helps researchers and students to appreciate certain qualities that significantly affects the life-sustaining capabilities of water. In this regard, it is imperative to appreciate the fact that the attraction created by a hydrogen bond is relatively strong, measured at 10-40 kJ mol-1 (Watson, 2017).
It must be made clear that although the said chemical bond is weaker compared to a covalent bond, its strength is significantly greater compared to a Van Der Waal’s type of chemical bond (Watson, 2017). This insight into the strength of hydrogen bonds helps explain the specific heat capacity, cohesion, adhesion, density, high boiling point, and solvent properties of water. In other words, the hydrogen bonds present in liquid water are strong enough to ensure a stable framework while at the same time having certain properties that allow for the absorption of energy and dissolution of certain molecules.
Specific Heat Capacity
As a result of the relative strength of hydrogen bonds, liquid water has high specific heat. Thus, a relatively high amount of energy is required to heat a certain amount of liquid water. Water has a certain chemical property that requires a high level of energy to break the hydrogen bonds. Consider for instance the fact that to increase the temperature of 1 kilogram of water by 1 degree Kelvin requires 4200 joules of heat energy (Bhoi & Jaswal, 2014). As a consequence, water possesses a chemical property that makes it an ideal coolant (Bhoi & Jaswal, 2014).
In this regard, water is seen as an ideal fluid or liquid needed to sustain life. It is difficult to imagine the impact of an ecosystem wherein living organisms is breaking down after performing strenuous activities due to the absence of a suitable fluid needed to absorb heat energy. Thus, animals can run long distances without overheating. The cooling capability of water is also manifested during the evaporation process, especially in the fact that it requires a great deal of energy to transform liquid water into gaseous form. As a result, heat transfer during the process of sweating and perspiration enables the body to release heat and protect itself from overheating.
Aside from the fact that the high specific heat capacity of liquid water makes it an ideal coolant for an overheated system, the chemical property made possible by the presence of stable hydrogen bonds allows for constant temperatures. This is a critical property because enzymes work best at optimum temperature ranges. Low temperatures cause sluggish enzyme activity. On the other hand, high temperatures degrade enzymes. Also, human babies inside the wombs of pregnant mothers do not possess temperature regulators. Thus, the amniotic fluid that is made up of liquid water provides an environment that is characterized by constant temperature levels.
Adhesion and Cohesion
The electrostatic force created by two atoms of hydrogen connected to one atom of oxygen enables water molecules to develop a significant number of hydrogen bonds. It is the presence of a significant number of hydrogen bonds that allow for the “sticky” property of water. Thus, water molecules stick to other water molecules and different surfaces. The presence of numerous hydrogen bonds in liquid water enables the liquid to experience a capillary effect as evidenced by the movement of water through a plant’s xylem. The adhesion effect created by water molecules as it travel through the xylem tubes also causes a cohesion effect that ensures the continuous flow of water (Song et al., 2014).
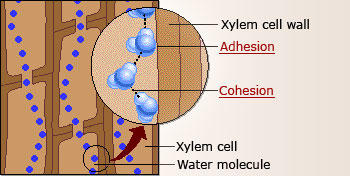
Plants are unable to absorb nutrients from the soil without the adhesion and cohesion capability of water molecules. The failure to absorb the said nutrients makes it impossible for plants to produce the food required to produce fruits and starches that are needed by animals and human populations. In other words, root crops, cereals, and fruits require the adhesion and cohesion properties of water.
Density of Water
As mentioned earlier, the design of the water molecules played a critical role in the development of the hydrogen bond. It has to be made clear that the design reveals two hydrogen atoms linked to one oxygen atom. In the case of water molecules in liquid water, the presence of numerous hydrogen bonds is made possible by the two lone pairs of electrons present inside an oxygen atom (OMICS International, 2014). In other words, the two lone pairs of electrons are like open terminals ready to connect with another positively charged hydrogen atom.
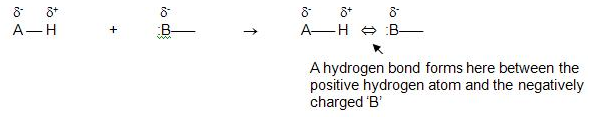
The said chemical property does not only allow the creation of numerous hydrogen bonds, it also affects the crystal structure of ice. The result is an open hexagonal lattice (OMICS International, 2014). This structure is also known as a 3-dimensional tetrahedral shape and made possible by the presence of two hydrogen bonds in every single water molecule. Thus, water expands when subjected to freezing temperatures. However, due to its unique structure, ice is less dense compared to liquid water.
Since ice is less dense than liquid water, frozen water floats on top of the liquid water as manifested in a frozen pond. At first glance, the fact that ice cubes float in a cup of liquid water seems insignificant. However, after closer scrutiny, researchers discovered that a pond or lake will never freeze into one solid block of ice even during winter months due to the unique property of liquid water. As a result, marine species at the bottom of the pond or lake can survive the winter months because of the said phenomenon. The absence of hydrogen bonds makes it impossible to develop this unique chemical structure and as a consequence marine species may never survive the winter season (Leung, 2014).
Solvent Properties
Salt and other nutrients have polar properties that make it easy for these substances to dissolve in water. These substances transform into ions in liquid solutions and easily attracted to the polar water molecules (Watson, 2017). As a result, it is relatively easy for water molecules to surround and disperse the said dissolved substances. The ability of water molecules to dissolve the salt and similar substances makes it an ideal transporting mechanism. Biological studies confirm the necessity of an efficient transport system to sustain life on planet Earth. The absence of an effective transport mechanism based on the properties of liquid water makes it impossible to nurture and replenish cell growth. Organs are going to fail without the transport system seen in blood flow and excretory processes.
Boiling Temperature of Water
It is of crucial importance for liquid water to have a high boiling point. Carbon dioxide for instance boils at very low temperatures. On the other hand, water boils at a hundred degrees Celsius. It requires a lot of energy to boil water. Thus, liquid water provides a stable and hospitable environment to various forms of living organisms (Watson, 2017). For example, the oceans are stable and hospitable because even if these oceans are exposed to the sun’s rays, water does not boil easily. One can just imagine the catastrophic consequences if water boils easily. Once again, it is the presence of the hydrogen bonds in water that makes it difficult to disrupt the cohesive bonds between water molecules.
It is imperative to have a high boiling point to maintain the liquid form of water in various conditions. Water in a frozen state is useless in sustaining life. Water has to exist in liquid form to experience the benefits described earlier, specifically the ability of liquid water to dissolve and transport materials.
Conclusion
Hydrogen bonds are relatively strong enough to allow the manifestation of certain chemical properties like high boiling point and high specific heat. However, the bonds are not as strong as a covalent bond, therefore, it allows a certain degree of flexibility as exhibited in the adhesion and cohesion of water through a plant’s xylem tubes. It is the hydrogen bonds that allow for the density of ice. In the interaction between the two states of water, liquid water is denser than frozen water allowing for the survival of marine life during the winter season. Also, it is the presence of hydrogen bonds that allow for the relatively easy dissolution of certain substances and therefore ensures life on planet Earth. As a result, biological organisms can reproduce and support life due to the presence of liquid water. Liquid water is stable due to the presence of hydrogen bonds.
Reference List
Acta Crystallographica 2017, ‘Hydrogen bond considerations’, Web.
Bhoi, R & Jaiswal C 2014, ‘Performance evaluation of heat transfer rate’, International Journal of Engineering Innovation & Research, vol. 3, no. 5, pp. 578-580, Web.
Leung, K 2014, ‘The influence of ceramic far-infrared ray irradiation on water hydrogen bonding and its related chemo-physical properties’, Hydrology: Current Research, vol. 5, no. 174, Web.
OMICS International 2014, ‘Hydrogen bond’, Web.
Song, Y 2014, ‘Characteristics of hydrogen bond revealed from water clusters’, European Physics Journal of Development, viewed 25 Jan. 2017, via EPJ database.
Watson, R. (2017). ‘What if there were no such things as hydrogen bonds?’, Young Scientists Journal, Web.