Abstract
The lab report helps to understand the science and techniques that are used to identify different types of bacteria based on their DNA sequences. DNA sequencing which was a time-consuming and complicated effort earlier is now much simpler with the wide array and range of equipment available. The paper provides a lab report on the method used for DNA sequencing of 16S rDNA which causes infectious diseases.
Introduction
Facilities available at the online lab allow the DNA sequence of the gene to be sequentially mapped (HHMO, 2007). The piece of DNA used for identifying bacteria is the region that codes for a small subunit of the ribosomal RNA (16S rRNA). Different bacterial species have unique 16S rDNA sequences. The identification is done by matching the sequence from the sample against a database of all known 16S rDNA sequences available at NCBI (NCBI, 2007).
Method
There are two distinct phases in the method. The first is the preparation of the DNA sequence and matching it with the database of known DNA sequences.
DNA Sequence
To find the DNA sequence and perform the sequence analysis, there are six distinct steps to be followed and are Sample preparation, PCR Amplification, PCR Purification; Sequence Preparation; DNA Sequencing, and Sequence Analysis.
Sample Preparation: In this step, a single colony of the bacterium is a culture dish and put into a microcentrifuge tube. The process of extracting bacterial DNA consists of dissolving the cell wall with a digestive buffer. The buffer contains proteolytic enzymes that “eat” the cell wall and this step may take a few hours. The proteolytic enzymes have to be denatured by heating the sample in a water bath at 100 degrees centigrade. the cellular debris is spun down in the centrifuge and appears as a solid deposit (pellet) at the bottom of the tube. The DNA is contained in the supernatant (the liquid), which is then transferred to the PCR tube. Given below is a screenshot of the process
PCR Amplification: To prepare the polymerase chain reaction (PCR), the PCR Master Mix solution is added to the sample DNA. The PCR Master Mix solution contains water; a buffer to keep the mixture at the correct pH for the PCR reaction; large quantities of the four nucleotides adenine, cytosine, guanine, and thymine; large quantities of oligonucleotide DNA primers that bind the 16S rDNA region to initiate the replication process; and a heat-stable DNA polymerase that extends the copy DNA strand. Once the reaction tubes are loaded onto the thermocycler, the automatic process of DNA replication starts

PCR Purification: The tube will now have many copies of 16s rDNA, each about 1,500 base pairs (bp) long. It is recommended to run a gel to confirm that the PCR reaction worked. The gel should contain three lanes: one for the negative control (i.e., water), which should not have a product unless the water was contaminated; another for the positive control (PCR product of a known DNA sequence) to make sure that the PCR itself worked; and the last lane for the sample. Once the PCR product is in the gel, the PCR product should be cut and the DNA should be isolated from the gel. Nowadays, you can buy compact microfilters to filter the DNA from the PCR tube without running a gel. A micro concentrator column is used for the process.

Sequencing Preparation: The DNA sample has been purified; the PCR tube should now contain only copies of the 16S rDNA and it can be taken to prepare the sample for automatic sequencing. A set of 12 primers; six for each strand of the double-stranded DNA are used. With multiple primers, short, overlapping stretches of DNA are sequenced to obtain the complete sequence. In addition, it is not necessary to sequence both strands, although sequencing both strands generates redundant data, thereby reducing error. The exact number and location of primers used in a reaction depend on the availability of suitable primers. The primers used here are available from a commercial source and bind to conserved regions of the 16S rDNA gene. They should thus be able to bind to the sequence regardless of the bacterial source.
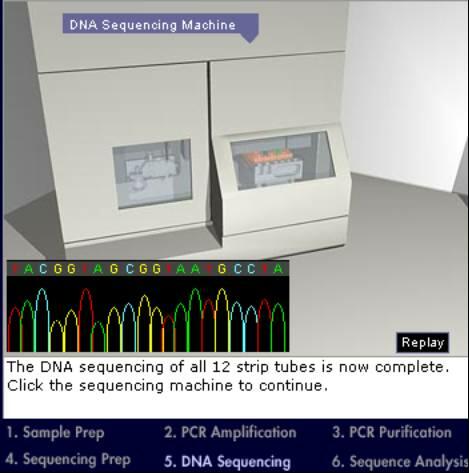
DNA Sequencing: In the previous step, 12 tubes contain the final PCR product, a mix of DNA pieces of variable length. All DNA pieces in each tube start with the same primer but end with a different nucleotide tagged with a fluorescent marker (different color for each nucleotide A, T, G, C). Individual DNA pieces have to be separated and the end nucleotide has to be identified. This is done by using an automatic sequencer that performs gel electrophoresis on the DNA in each tube. Gel electrophoresis is a method to separate molecules based on differences in size. The sequencer has a thin capillary tube attached at one end to a syringe mechanism that contains a buffer solution. The tube is filled with the buffer solution and the other end inserted into one of the tubes containing the DNA pieces. Then, an electric current is applied so that the end of the tube in contact with the DNA has a negative charge and the syringe ends a positive charge. Since DNA molecules are negatively charged, they start to move through the tube toward the positively charged syringe end, with the smaller pieces moving faster than the larger ones. Near the syringe end, the capillary tube passes through a laser beam that excites the fluorescent markers, and optical detectors detect the color of the fluorescence.
DNA Sequence Analysis: After the sequence information has been gathered from all the reaction tubes, the computer builds the actual sequence by matching together different pieces. The 16S rDNA sequence is available for this bacterial species, which can be compared with all other known 16S rDNA sequences for identification.
The data output of the DNA sequence is shown below.

Select the full sequence and copy it.
Matching the DNA Sequence
After copying the DNA sequence, open the BLAST site of NCBI. Paste the sequence in the search window and click the BLAST button. The full DNA sequence can be seen.
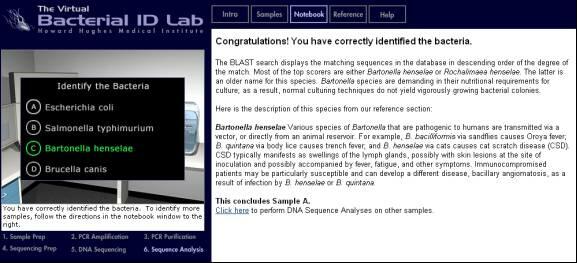
Results and Conclusion
The bacterium has been correctly identified as “Bartonella henselae”
References
BLAST. (2007). BLASTN programs search nucleotide databases using a nucleotide query. Web.
HHMI.(2007). Online MedLab.
NCBI. (2007). DNA Sequence Database.