Introduction
- Radionuclide is a radioactive isotope of elements.
- Radionuclides of heavy-metal elements such as uranium, thorium, cesium, have longer half-lives.
- They are produced in nuclear reactors, by the oil and gas industry, and by nature.
- Prolonged exposure to large doses is harmful.
- This research studies kinetics of radionuclides in UAE soil using radio analytical and plasma mass spectrometry (Martin 21).
Radionuclide is a radioactive isotope of elements with an atom having extra nuclear energy, which makes it unstable. All elements can have radionuclides and an example is hydrogen that has a radionuclide tritium. Radionuclides of heavy-metal elements such as uranium, thorium and cesium, have longer half-lives. These are produced in nuclear reactors, particle accelerators, cyclotrons, radio imaging and medical scanning devices, by the oil and gas industry, and they also occur in nature. Large and continuous exposure to nuclear radionuclides is harmful to living organisms (Martin 21). This research studies the kinetics of leaching of long lived radionuclides from UAE urban soil samples using radio analytical techniques and plasma mass spectrometry.

Aims and Objectives
- Aim: To study kinetics of leaching of radionuclides in UAE urban soil.
- Objectives are to study:
- Extent and impact on the environment;
- Generate data on leaching;
- Effect of pH on metal leaching and methods of control;
- Learn about NORMS and TNORMS.
Aim of the research is to study the pattern and the kinetics of leaching of long lived radionuclides from UAE urban soil samples using radio analytical techniques and plasma mass spectrometry.
Objectives of the research are:
- To identify the extent and possible impact of radionuclide/ metal leaching on the environment.
- To generate data of the leaching process and the kinetics of leaching and to add to the scientific knowledge in those areas pertaining to the UAE.
- To assess the effect of pH on metal leaching with a view to find possible solutions to control/ curtail the harmful effects of leaching.
- To contribute to the body of knowledge about the Naturally Occurring Radionuclides (NORMS) and Technically Enhanced NORMS (TNORMS) data for the UAE.

Research Questions
- Research questions proposed are, what are:
- Current levels of NORMS and TNORMS in UAE soil.
- What type of data will be generated?
- What scientific knowledge can be developed
- What indicators can be developed?
- How can data help to find solutions?
- The problem statement is: “How can research show levels of long lived radio nuclides?
Research questions proposed are:
- What are the current levels of Naturally Occurring Radionuclides (NORMS) and Technically Enhanced NORMS (TNORMS) in UAE soil?
- What type of data will be generated by the leaching process and the kinetics of leaching studies, and what scientific knowledge can be developed on radionuclides metal leaching from the soil?
- What indicators can be developed to assess the extent and impact of radionuclide/ metal leaching on the environment?
- How can data on the effect of pH on metal leaching be used to find possible solutions to control/ curtail the harmful effects of leaching?
The problem statement is:“How can the leaching studies and the radiation measurements give an indication of the level of long lived radio nuclides that can migrate from the soil to the bore hole water and agricultural run-off in UAE?”

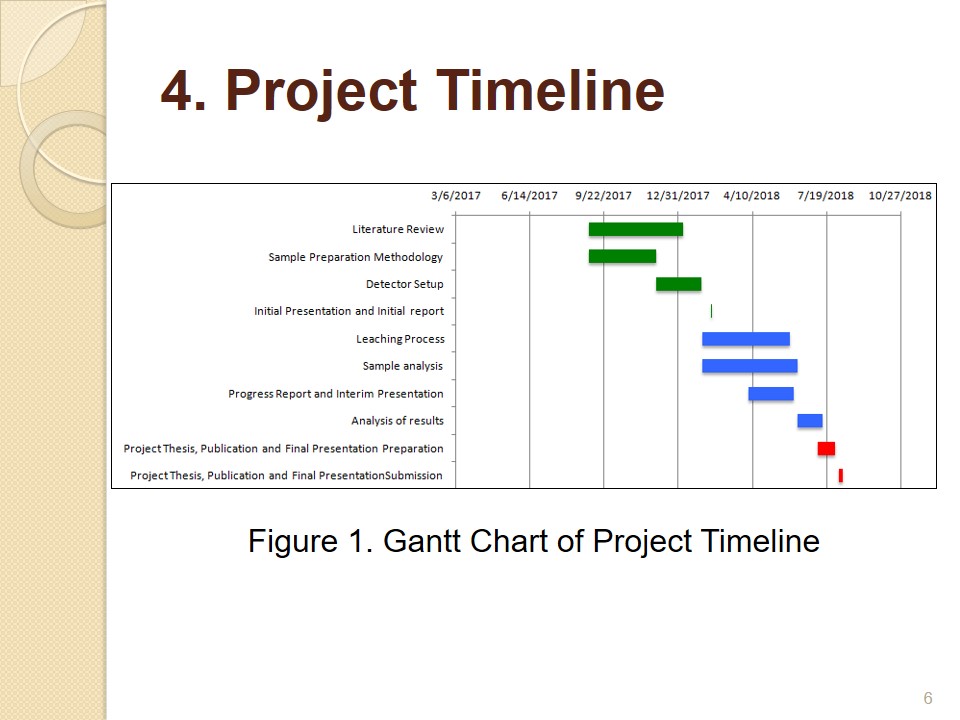
Project Timeline
The project time line with a list of major milestones and their schedule is illustrated in the above Gantt chart.
Types of Radionuclides
- Four main classes: cosmogenic, primordial, anthropogenic, and natural decay series.
- Primordial have very long half lives, formed when earth was created.
- Natural decay series formed by decay of primordial series.
- Cosmogenic formed from cosmic radiation.
- Anthropogenic radionuclides created by humans (Sharma et al. 951).
As per their origin, radionuclides are present in four main classes in the environment. These are cosmogenic, primordial, anthropogenic, and natural decay series. Primordial radionuclides were developed when the earth was formed and they have very long half-lives. Some of these are 40K with a half-life of 1.248 x 109 years, 232Th with a half-life of 1.405 x 1010 years, and 238U with a half-life of 4.468 x 109 years. Natural decay series are formed by the decay of primordial radioactive isotopes and some examples are 232Th, 235U, and 238U. Cosmogenic radionuclides are formed due to cosmic radiation and some examples are 14C and 10Be. Anthropogenic radionuclides are formed through nuclear power plants and weapons testing, nuclear fuel processing, dumping of fissile material and equipment, industrial activity related to oil and natural gas extraction, and other such activities. Some examples are 131I. and 137C (Sharma et al. 951).

Sources of Radionuclides
- Natural sources are cosmic radiation, radon gas, drinking water.
- Soil around nuclear reactors, power plants and in zones around crude oil fields have radionuclides.
- Soil and air around nuclear reactors is monitored for radionuclides.
- Essential to protect humans and animals (Martin 28).
Natural sources of radionuclide are from radon gas found in certain rocks, from cosmic radiation, from drinking water and soil ingredients, and in areas near nuclear reactor sites and power plants. Generally, areas around nuclear sites are monitored for radionuclides in drinking water, soil, and in plants. High levels of radionuclides from activities such as oil and gas, phosphate mining, commercial and shipping activities tend to increase the level of radionuclides. The Middle East has not been well mapped to understand the distribution and presence of radioactive concentration (Martin 28).

Contamination Methods
- Nuclear accidents cause immediate contamination of soil, air, and water.
- Radionuclides leach from water of nuclear plants to the soil.
- Airborne particulate matter is sorbed by soil particles.
- Large amounts of leaching cause cancer and other problems. (Al Shamsi 15).
While a nuclear accident would immediately render the accident area unsafe, danger is present from gradual leaching of radionuclide from water used in the nuclear power plants, from contamination and from other pathways, into the soil and water system. Other pathways are airborne particulate matter that is emitted from nuclear power plant stacks. Some areas of the Middle East such as Kuwait have seen testing for radionuclides in sand and soil. This area had seen intense fighting in 1991 during the Gulf War and hundreds of oil wells were set on fire or damaged, causing huge oil spills that formed large surface deposits. The war also saw the use of more than 800 tons of depleted uranium shells for missiles (Al Shamsi 15).

Pathways
- Radionuclides adsorbed by soil, washed with rain.
- Enter water streams, lakes, ponds, underground waterways.
- Enter plants through roots and deposited in leaves, stems, fruits, poison humans and animals.
- Amount depends on solubility, sorption capacity of soil. (Al Shamsi 29).
These radionuclides are adsorbed by the soil and washed down due to precipitation and rain, and enter underground water streams. Plants and vegetables that consume these waters also imbibe the radionuclides, which enter the plant system and they are deposited in the leaves, stems, flowers, and fruits. The soil-plant pathway allows for long-lived radionuclides to enter into fruits and flowers. The amount of radionuclides depends on the concentration of radionuclides in the soil, their leachability and solubility, and the sorption capacity of soil particles (Al Shamsi 29).

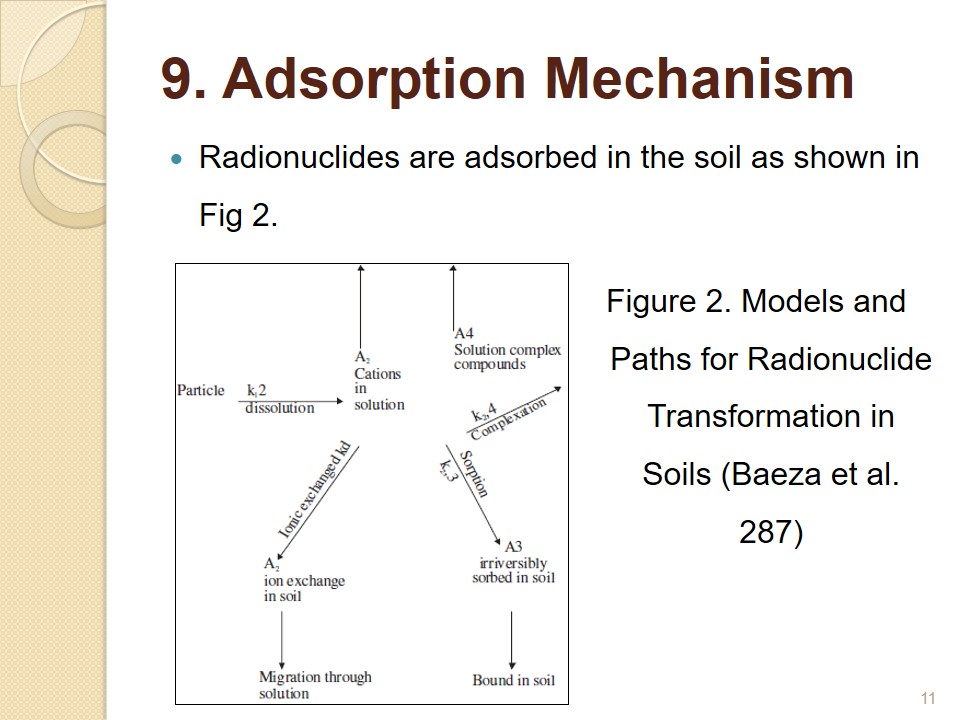
Adsorption Mechanism
- Radionuclides are adsorbed in the soil as shown in Fig 2.
Radionuclides are adsorbed in the soil as shown in Fig 2. Cesium is positively charged ion and it is adsorbed by soil colloids, and uranium radionuclide UO2+ is also positively charged and adsorbed. However, further pathway and entry into the plant depends on the pH value and organic content of the soil. Residence times of radionuclides vary and the migration from lower to upper levels takes a certain time, depending on the porosity, soil activity and disturbances, and other factors. The speed at which radionuclides are adsorbed depends on the alkalinity and moisture content of the soil. Soils that are alkaline and have higher moisture content have higher adsorption qualities (Baeza et al. 287).
Leaching by Soil-Root Pathway
- Colloids and organic material are permeable and adsorption is high.
- Sand and pebbles are not permeable.
- Clay attracts +ions and exchange frequency increases.
- Same ambient conditions cause increase in exchange of cations. (Igwe et al. 1543).
Colloids or clay minerals, organic matter, and humus are important channels, since they are permeable, they have a higher surface area, and they have a higher chemical activity. Silt, sand, pebbles and rocks are hard, and not permeable. Clay has molecules with negatively charged oxygen atoms at the top and lower surface. These clay particles attract the positive ions of H+, Na+, Ca2+, and other elements and compounds. Cation exchange theory helps to understand the replacement order, where cations replace other ions. When the same ambient conditions exist, the exchange frequency rises with more valence of exchange cations, so Na+< Ca2+< Al3+<Th4+, and other exchanges occur (Igwe et al. 1543).

Mechanism of Soil Root Pathway
- Radiation enters plants through the soil and roots.
- Then it is transferred to fruits, leaves and other parts.
- Particulate radionuclides in solid form are not easily soluble
- They are leached gradually. (Al-Masri et al. 325)
Soil-root pathway is important in transfer of radionuclide into fruits, since it is available for all plants growing in an area with increased nuclear radiation. Particulate radionuclides, deposited into the soil are usually not easily soluble in water. Rather, they are leached gradually through a weathering process. The equation to describe the flux of radionuclides leached into soil is (Al-Masri et al. 325; Huy and Luyen 333; Chkir et al. 652):
dQ/dt = – kwQ-kpQ
Where:
- kw is the leaching rate from weathering T-1;
- kp is the physical decay rate;
- Q is the amount of radionuclides deposited in the soil, calculated as unit/ L2;
When radionuclides are leached into the soil are sorbed by the particles, equilibrium of distribution is defined between the soil solution and soil particles, and this is given by the expression:
Cp – Kd Cs
Where:
- Kd is distribution coefficient L3/ M;
- Cp is radionuclide concentration in soil solution, unit/ M;
- Cs is the radionuclide concentration in the soil solution, unit/ L3;
The total radioactivity/ unit area of the ground Ctotal is given as:
Ctotal = D(m +BdKd) Cs
Where:
- D is depth of root zone, L;
- m is the volumetric content of moisture in soil;
- Bd is the bulk density of soil M/ L3;
Assuming that absorption by plants is proportional to the plant weight and size and soil solution, and then the relation is.
d (p x fg)/ dt = kb x fg x Cs – kp (P x gf).
Where:
- P is the radionuclide concentration in plant, Unit/ M;
- fg is the function of plant growth (M/ L2 at time t);
- kb is the uptake rate constant of plant (L3/ M/ T).
Safe limits
- Safe exposure level is not clear.
- Value varies among countries.
- UNSCEAR indicates different levels for cosmic radiation, external terrestrial radiation, inhalation exposure, and total ingestion.
- Higher long-term exposure leads to cancer and other diseases of kidneys, lungs, etc. (United Nations Scientific Committee on the Effects of Atomic Radiation 140).
The safe or permissible level of radionuclides in soil, water, food, and various parts of the body is not clear. The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) provides a list of tables giving the level of exposure to different levels of natural radiation in different regions and countries. The level of exposure from cosmic radiation is 0.3-1.0 mSv (millisievert), for external terrestrial radiation, it is 0.3-0.6 mSv, for inhalation exposure it is 0.2-1.0 mSv, and for total ingestion it is 0.2-0.8 mSv. Exposure of 100 mSv/ year can lead to cancer (United Nations Scientific Committee on the Effects of Atomic Radiation 140).
Radiation units: SI unit Becquerel Bq; radiation absorbed dose unit nGy (Martin 39).

Soil Radiation in UAE
- UAE constructed four nuclear power plants, operational by 2020.
- UAE has a huge oil and gas industry that emits radionuclides.
- Scanty research on the problem.
- Research on levels and leaching mechanisms in soils will be done in this study. (Murad et al. 6159).
UAE has constructed four nuclear power plants at Barakah, and the plants will be operational by 2020. However, UAE has a huge oil and gas industry, and this industry has emitted large amounts of radionuclides over the past few decades. In addition, there is radiation from natural sources (Murad et al. 6159). Published research on assessing the levels of radionuclides concentration in UAE is scant. Researchers have presented studies on the concentration of gross β and α in radionuclides in soil and water. The researchers collected samples of soil and water from different locations and tested them using standard lab equipment. Research on the concentration of radionuclides and leaching mechanisms in soils at different pH values is not available. This research aims to fill this gap .

Literature Review
- Research done to examine Uranium and Thorium leaching in France.
- Gamma spectrometer used to detect radionuclides in water, soil, and leaves.
- Preferential leaching of Th from shallow regions due to the higher presence observed
- Acido-complexolysis weathering methods observed
- Leaching for several centuries observed. (Rihs et al. 7710)
- Research to study radioactivity in the Gulf of Aqaba, Jordan.
- Water samples collected from different depths, and from beach sediments.
- Samples analyzed with gamma spectrometer.
- Radionuclide concentration from seafloor and beach twice than those from other locations. (Ababneh et al. 295)
- Research undertaken to study gamma-emitting radionuclide concentration in palm dates from UAE.
- Samples taken from 10 types of palm dates.
- Examined for radionuclides of thorium, uranium, and others.
- Samples tested with gamma spectrometer.
- Results showed significant levels of radionuclides. (Rahman and Solodov 467)
- Tests done on sand samples in North Kuwait that saw fighting during the Gulf War.
- Samples collected and analyzed in US, using gamma spectrometer.
- Testing was done for isotopes of Thorium, Cesium, Radium, and others.
- Concentrations were below international levels.
- Low level due to impermeable nature of sand that prevents leaching (Jallad 70).
- Study done to examine effect of pH on radionuclides in soils.
- Leaching depends on the soil redox potential and when water is present.
- Results show leaching is steady in neutral pH
- Higher with low pH sandy soils, slower in higher pH soils.
- Since desert has low moisture, leaching is reduced (Koch-Steindl and Pröhl 96).
- Research to examine inverse relation of radionuclide leaching rate and pH level.
- Soil samples collected from 20 quarries in Nigeria and checked for K, U, and T radionuclides.
- Relation examined with particle size, organic matter, carbon, and pH values.
- pH meter and gamma spectrometer used to measure samples.
- Increase in radionuclide seen with pH values below six (Okedeyi and Gbadebo 695).
- Research conducted in Forsmark, Sweden, to see if contaminated soils can be used for agriculture.
- Samples collected from different depths along with pore water.
- Samples processed and analyzed with inductively coupled plasma sector field mass spectrometer.
- Concentrations of radionuclides increased when pH values were below 5.5 and decreased for 8-8.5 pH. Such soils will be difficult for cultivation. (Sohlenius et al. 418)
Rihs et al. examined the chemical kinetics and dynamics in soils of France for the Uranium and Thorium series (7707). Gamma spectrometer was used to find the levels of 238U, 230Th, 226Ra, 232Th, 228Ra, and 228Th in water seepage, solid particles, and mature leaves of beech trees. Ra and Th transfer follow the protocols of Acido-complexolysis weathering methods. Preferential leaching of Th from shallow regions due to the higher presence of organic colloids that started about 18 years back was observed. There was a continuous increase in the pH level over the previous two decades. A soil horizon of lower values was impacted by the preferential leasing of Th. A migration of Th isotopes that lasted for several centuries existed and this helped to understand the colloidal migration kinetics (Rihs et al. 7710).
Ababneh et al. studied the concentration of radioactivity concentrations in the Gulf of Aqaba, Jordan (292). The study was to establish a baseline data and understand the impact on the marine environment. The researchers studied the radioactive levels of gamma-emitting radionuclides in beach and core sediments of the Gulf of Aqaba regions in five regions. Water samples collected from 5, 15, and 35 meters along with beach sediments. Samples frozen, sliced, crushed, and tested with gamma spectrometers. Concentrations of 238U, 235U, and 226Ra for seafloor and beach sediments from the areas of phosphate rich zones, had levels that were twice higher than the values from other locations. Level of uranium concentrations showed less variation at 15 cms (Ababneh et al. 295).
Rahman and Solodov carried out a research to develop a radiation baseline in UAE (465). Gamma-emitting radionuclide concentration in palm dates, from trees growing in the UAE region studied. Samples from 10 types of palm dates were obtained for analysis. They examined naturally present radionuclides such as 232Th, 238U, 40K, 137Cs, and others. Samples prepared by crushing and oven drying, and then tested using a gamma spectrometer. The spectrometer measurement showed concentrations of 0.61-0.89 Bq Kg-1 for 238U, 0.10-0.23 Bq Kg-1, for 232Th and 191-362 Bq Kg-1 for 40K. Wet conditions concentrations were also measured and they showed values in the same range, though the variation was less (Rahman and Solodov 467).
Jallad carried out a study to find the radioactive characterization in North Kuwait that saw intense fighting during the Gulf War (69). Samples were conducted from 16 sites at depths of 5 cms, and sent for analysis in US. The samples were dried in an oven at 103 degree centigrade, pulverized, and homogenized, and checked with a gamma spectrometer. Samples were checked for U-238, Ra-226, K-40, Th-232, Cs-137 and I-131. Spectrum of the samples was obtained and the gamma photo peaks were measured. Radionuclide concentration and Minimum Detectable Concentration (MDC) was measured using the spectrometer. Findings indicate that Uranium-238 level was 11Bq/kg, Thorium-232 was found to be 30 Bq/kg, Radium-226 was 12 Bq/kg, Potassium-40 was 397 Bq/kg, Cesium-137 was 2.0 Bq/kg, and Iodine-131 stood at 19.0 Bq/kg. Conclusion is that sand is impermeable and leaching is low. Hence, the levels were below the expected concentrations (Jallad 70).
Koch-Steindl and Pröhl examined research to understand leaching effect of pH on radionuclides in soils (94). Leaching is regulated by the redox potential and the soil pH. Eh-pH stability diagrams were used to assess the radionuclide behavior. Redox processes happen when water molecules are present. Stability of water depends on the decomposition of water into oxygen, while the lower limit depends on the decomposition of hydrogen. Leaching of radionuclides is steady at the neutral pH values, it is higher in sandy soils with low pH value, since sandy soils have larger concentrates of lime and carbonates, and leaching is slower in higher pH soils that have more clay. The absence of water and moisture in desert areas, reduce the leaching effects (Koch-Steindl and Pröhl 96).
Okedeyi and Gbadebo researched the inverse relation of radionuclide leaching rate and pH level (693). They collected soil samples from 20 quarries in Nigeria and measured the presence of 40K, 228U, and 232T radionuclide’s and the relation with particle size, organic matter, carbon, and pH values. The samples were gathered from various depths, air dried, pulverized and passed through a sieve of 2 mm mesh size. pH was measured by mixing 2 grams of the samples with 20 ml of distilled water and left for 10 minutes to settle. The mixture was then stirred and allowed to stabilize for 30 minutes. A pH meter was used to measure the pH and gamma spectrometer radiation, and the results analyzed. It was seen that with increase in radionuclide levels, pH values fell to below six (Okedeyi and Gbadebo 695).
Sohlenius et al. conducted a research to understand the mobility of different elements that affect the leaching and movement of radionuclides (416). Objective was to see if contaminated soil could be used for agriculture. Soil samples were collected from Forsmark, Sweden from depths of 20-25 and 50-55 cm. Pore water and solid phase samples were collected. Samples were digested with aqua regia and analyzed using inductively coupled plasma sector field mass spectrometer. pH value and mineral content was examined. Results indicate that concentrations of Cesium, Ni, U and R increased when pH values were below 5.5, and decreased for 8-8.5 pH. Such soils will be difficult to use for cultivation (Sohlenius et al. 418).







Methodology
- Samples from a previous study will be processed and leached.
- Gamma-ray instrumentation and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) will be used.
- Toxic level estimation of arsenic, lead, and mercury will be carried out by ICP-MS equipment. (Al-Ali 41).
In a previous research, soil samples were collected from urban areas of UAE. These will be processed and leached in this research (Al-Ali 41). These samples will be now be leached at the university laboratory. Modern gamma-ray instrumentation and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) will be used for leaching. Samples will be segregated as per the depth at which they were collected and the pH value. A previous study done in UAE will serve as the model for the leaching process and kinetic study. Toxic level estimation of arsenic, lead, and mercury will be carried out by ICP-MS equipment.
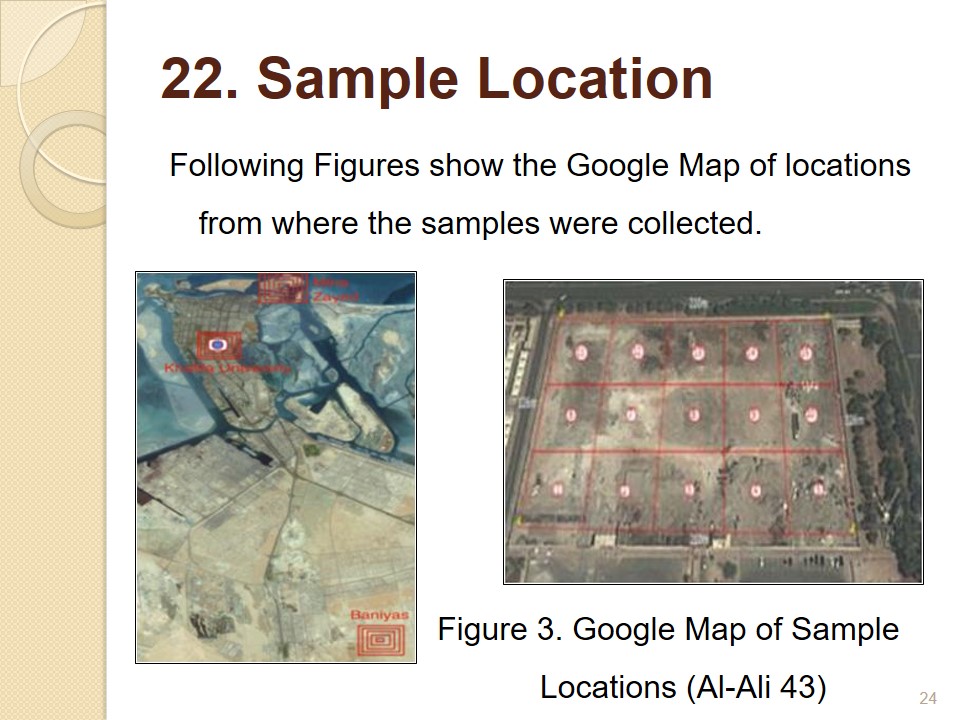
Sample Location
Following Figures show the Google Map of locations from where the samples were collected.
Samples were collected in a previous research from different locations in UAE, and these will be processed and leached in this research. The Google Map in Fig 3 was prepared to cover the area under study. Location coordinates of each sampling area were noted down, so that it will be easy to pinpoint the exact location from which a sample was taken. A hand operated sampling drill was used to dig into the earth and five grams of each sample was collected in polypropylene containers (Al-Ali 43). Fig 3.2 illustrates the Google map of the locations.
Sample Preparation
- Samples will be leached for 12-16 weeks.
- De-ionized water of acidic, neutral, and basic will be used for evaluation.
- Leachate samples will be collected weekly and analyzed with ICP-MS.
- Samples will be acid washed and mixed with an orbital shaker. (Al-Ali 21).
The following radionuclides were found in the previous research 228AC, 212Pb, 212Bi, 208Ti, 226Ra, 214Pb, 214Bi, and 40K (Al-Ali 46). In this research, the samples will be leached for 12-16 weeks. De-ionized water of three pH levels, acidic, neutral and basic, will be used to evaluate the effect of pH on metal leaching from soil samples. Leachate samples will be collected every week and analyzed with the ICP-MS. To avoid contamination, special polypropylene tubes will be acid washed to get rid of all surface contaminations prior to the leaching process. The samples will be stored at room temperature and mixed using an orbital shaker (Al-Ali 21).

ICP-MS – Instrument
- Inductively Coupled Plasma Mass Spectrometer (ICP-MS) to identify and quantify isotopes from samples.
- Wide detection limits with ppt levels for elements.
- Higher throughput for fast sequential scanning.
- Multiple element detection in single scan.
- Detects individual radionuclides and prints results as reports. (Thomas 58).
Inductively Coupled Plasma Mass Spectrometer (ICP-MS): ICP-MS is an instrument which will help to identify and quantify different isotopes of elements in the samples. This study uses the PerkinElmer DRC-e model. The instrument has wide detection limits that extend to the ppt level for certain elements, with higher throughput for fast sequential scanning. The instrument detects multiple elements in a single scan. Additional features such as collision/ reaction cell to minimize matrix interferences, extends the capabilities of the device (Thomas 58).
Liquid Scintillation Analyzer
- Tri-Carb® 3110TR instrument to detect alpha, beta, gamma radioactivity and luminescence.
- Allows beta particle and photon production and reduction.
- Cocktail prepared, substrate dissolved to produce Ultima Gold AB.
- Solution agitated and examined for background count, and to calculate alpha and beta efficiency. (Dias et al.3).
The Tri-Carb® 3110TR instrument is a liquid scintillation analyzer used to detect alpha, beta, gamma radioactivity and luminescence in samples. The instrument operates with processes such as beta particle and photon production and reduction. The instrument has several features that provide for the lowest backgrounds with replay sample recall and reprocessing, which reduces lost data. It is used extensively for advanced analysis and studies for 1, 2, and 3 radionuclides. Cocktail prepared with 100 ml of samples and evaporated without boiling to achieve dryness. Substrate dissolved in 10 ml of 0.1M nitric acid to produce Ultima Gold AB. The solution was transferred to a vial and agitated for 3 minutes. Counting was carried out for a 0-2000 channels and pulse shape determination was set at 128. Alpha efficiency was determined with a 237Np standard and beta efficiency was determined with 90Sr/ 90Y. Packard TRICARB 2700 LSC System was used to determine the background count. Standard equations were used to calculate the alpha and beta efficiency (Dias et al.3).

Pictures of Instruments

Works Cited
Ababneh, Zaid, et al. “Assessment of Gamma-Emitting Radionuclides in Sediment Cores from the Gulf of Aqaba, Red Sea.” Radiation Protection Dosimetry, vol. 141, no. 3, 2010, pp. 289-298.
Al-Ali, Abdulla. An Initial Radiation Baseline Study of Urban Environments in UAE. Thesis, Khalifa University, UAE, 2016.
Al-Masri, Mian, et al. “Transfer of 40K, 238U, 210Pb, and 210Po from Soil to Plant in Various Locations in South of Syria.” Journal of Environmental Radioactivity, vol. 99, no. 2008, 2008, pp. 322-331.
Al Shamsi, Dalal. Natural Radioactivity in Groundwater, Rocks and Sediments from some Areas in the UAE: Distribution, Sources and Environmental Impact. Dissertation, United Arab Emirates University, 2014.
Baeza, Ahamad, et al. “Seasonal Variations in Radionuclide Transfer in a Mediterranean Grazing-Land Ecosystem.” Journal of Environment Radioactivity, vol. 55, 2001, pp. 283–302.
Chkir, Noe, et al. “Uranium Isotopes in Groundwater from the Continental Intercalaire Aquifer in Algerian Tunisian Sahara (Northern Africa).” Journal of Environmental Rradioactivity, vol. 100, no. 8, pp. 649-656.
Dias, Fabiana, et al. “Total Alpha and Beta Determination by Liquid Scintillation Counting in Water Samples from a Brazilian Intercomparison Exercise.” Proceedings of International Nuclear Atlantic Conference 2009 Held 27 September – 2 October 2009 at Rio de Janeiro, Brazil, Brazilian Nuclear Energy Commission, 2009, pp. 1-8.
Huy, Nam,and Taim Luyen. “Study on External Exposure Doses from Terrestrial Radioactivity in Southern Vietnam.” Radiation Protection Dosimetry Journal, vol. 118, no. 3, 2005, pp. 331-336.
Igwe, John, et al. “Kinetics of Radionuclides and Heavy Metals Behavior in Soils: Implications for Plant Growth.” African Journal of Biotechnology, vol. 4, no. 13, 2005, pp. 1541-1547.
Jallad, Karim. “Radioactive Investigation of Sand from the Northern Region of Kuwait.” Environment and Natural Resources Research, vol. 3, no. 4, 2013, pp. 68-77.
Koch-Steindl, Hans, and Gans Pröhl. “Considerations on the Behavior of Long-Lived Radionuclides in the Soil.” Radiation and Environmental Biophysics, vol. 40, no. 2, 2001, pp. 93-104.
Martin, James. Physics for Radiation Protection: A Handbook. Wiley Publications, 2008.
Murad, Ahmad, et al. “Natural Radioactivity in Groundwater from the South-Eastern Arabian Peninsula and Environmental Implications.” Environmental Monitoring and Assessment, vol. 186, no.10, 2014, pp. 6157-6167.
Okedeyi, Akinya, and Oboje Gbadebo. “Effects of Physical and Chemical Properties on Natural Radionuclides Level in Soil of Quarry Sites in Ogun State, Nigeria.” Journal of Applied Sciences, vol. 14, 2014, pp. 691-696.
PerkinElmer. “Tri-Carb 3110 Liquid Scintillation Counter, TR Domestic Operation Manual.” PerkinElmer Inc, 2018. Web.
Rahman, Rubina, and Alexander Solodov. “Analysis of Natural and Anthropogenic Radionuclide Content in Palm Date Fruit of the United Arab Emirates: A Baseline Study.” Health Physics, vol. 111, no. 5, 2016, pp. 465-470.
Rihs, Sophie, et al. “Using Short-Lived Nuclides of the U-and Th-Series to Probe the Kinetics of Colloid Migration in Forested Soils.” GeochimicaetCosmochimicaActa, vol. 75, no. 23, 2011, pp. 7707- 7724.
Sharma, Sunita, et al. “Phytoremediation: Role of Terrestrial Plants and Aquatic Macrophytes in the Remediation of Radionuclides and Heavy Metal Contaminated Soil and Water.” Environmental Science and Pollution Research, vol. 22. no. 2, 2015, pp. 946-962.
Sohlenius, Gustav, et al. “Inferences about Radionuclide Mobility in Soils Based on the Solid/Liquid Partition Coefficients and Soil Properties.” Ambio, vol. 42, no. 4, 2013, pp. 414–424.
Thomas, Ron. Practical Guide to ICP-MS: A Tutorial for Beginners. 3 ed., CRC Press, 2013.
United Nations Scientific Committee on the Effects of Atomic Radiation. “UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes.” United Nations Scientific Committee on the Effects of Atomic Radiation, 2000. Web.