Objective
The objective of the experiment is to demonstrate how substitution reactions of alcohol occur. Specifically, the experiment aims to demonstrate the formation of 1-bromobutane from 1-butanol through a nucleophilic substitution reaction.
Physical Properties
1-Butanol
Form: Colorless liquid
Molecular formula: C4H10O
Molecular weight: 74.12
Melting point: -89°C
Boiling point: 117.6°C
Water solubility at 20°C: 80g/L
Density: 0.81g/mL
Hazards: Harmful, toxic, and highly flammable
Hydrogen Bromide
Form: Colorless liquid
Molecular formula: HBr
Molecular weight: 80.91
Melting point: -87°C
Boiling point: -67°C
Water solubility at 20°C: Miscible
Density: 1.49g/mL
Hazards: Corrosive and irritant
Sulfuric Acid
Form: Colorless oily liquid
Molecular formula: H2SO4
Molecular weight: 98.08
Melting point: 10°C
Boiling point: 290°C
Water solubility at 20°C: Miscible
Density: 1.84g/mL
Hazards: Highly flammable, corrosive, irritant, and toxic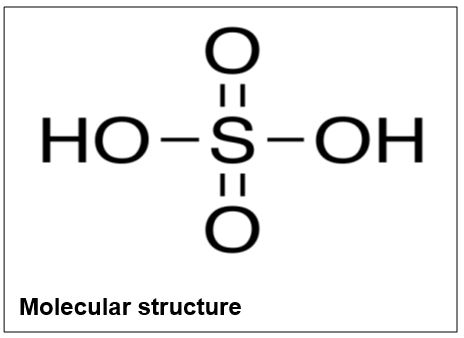
Sodium Bicarbonate
Form: White powder
Molecular formula: NaHCO3
Molecular weight: 84.01
Melting point: 300°C
Boiling point: 851°C
Water solubility at 20°C: 90g/L
Density: 2.16g/mL
Hazards: Irritant, and toxic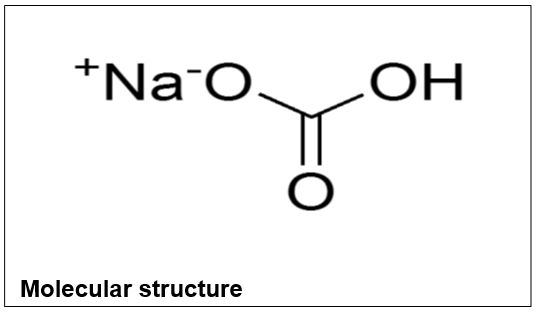
Sodium Sulfate
Form: White crystals or powder
Molecular formula: Na2SO4
Molecular weight: 142.04
Melting point: 884°C
Boiling point: 1700°C
Water solubility at 20°C: 18mg/L
Density: 2.68g/mL
Hazards: Irritant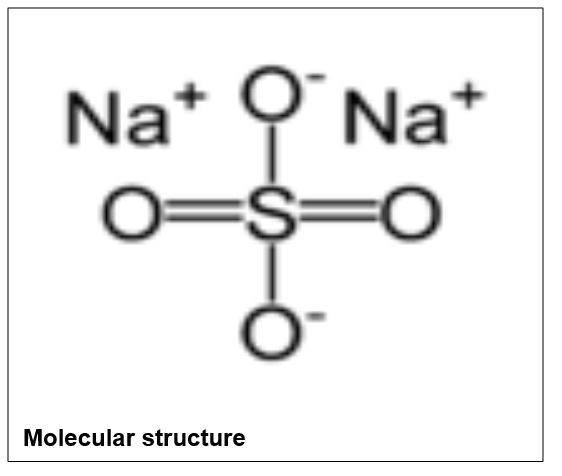
1-Bromobutane
Form: Colorless-yellow liquid
Molecular formula: C4H9Br
Molecular weight: 137.02
Melting point: –112°C
Boiling point: 100-101°C
Water solubility at 20°C: 0.608g/L
Density: 1.276g/mL
Hazards: Highly flammable, irritant, and dangerous for the environment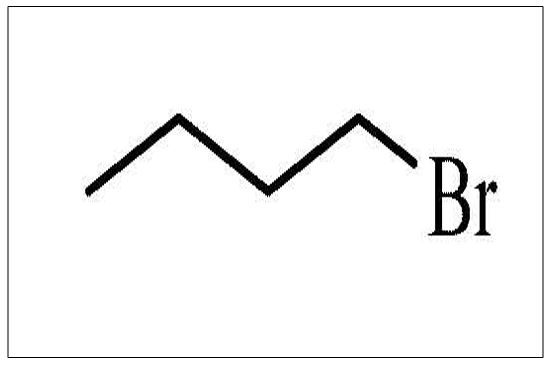
Procedure
To commence the experiment, 6.2ml of 1-butanol was measured accurately and was put into a 100ml round bottom flask. While swirling, 10 ml of 48% hydrogen bromide (HBr) was added to the round bottom flask containing 1-butanol. Subsequently, 4ml of concentrated sulfuric acid (H2SO4) was added to the flask slowly and cautiously while swirling the content and cooling the flask in an ice bath. Two boiling chips were added to the flask and then a reflux condenser was connected to the top of the round bottom flask.
Water hoses were connected to the reflux condenser, and a vacuum tube was connected on top of the condenser to absorb noxious fumes. The unconnected end of the vacuum hose was connected to the sink aspirator through the gas trap, and the aspirator was switched on and set to full blast. To test the functioning of the aspirator, a finger was placed on the open joint of the vacuum connecting tube, and slight suction was felt. The reaction mixture in the flask was then heated for 45 minutes at reflux.
After the heating duration of 45 minutes, the reaction mixture in the flask was allowed to cool in the ice bath. 10ml of de-ionized water was added slowly and cautiously to the mixture via the condenser. Another two boiling chips were added to the mixture, and simple distillation apparatus was set by disconnecting the vacuum from the apparatus. The distillation process was started, and the distillate was collected using a 25ml round bottom flask immersed in the ice bath. The distillation process was stopped when the temperature of the distillate reached 100C.
A transfer pipette was then used to remove the aqueous layer from the distillate and placed in another flask. Moreover, a transfer pipette was used to transfer 5ml of de-ionized water to the organic layer, and the aqueous layer was combined with the previous aqueous layer. The organic layer was also washed with 5ml of 5% sodium bicarbonate and the aqueous layer was obtained and combined with the previous aqueous layers in the flask.
Moreover, 5ml of de-ionized water was added to the organic mixture, and the aqueous layer was removed and combined with previous aqueous layers in the flask. The remaining organic layer was obtained and placed on anhydrous sodium sulfate to dry. To measure the organic product, the dried organic layer was decanted into a pre-weighed sample bottle and capped. To obtain the results, the product in the sample bottle was weighed, IR determined, and percent yield calculated.
Results
Theoretical yield
Mass of 1-butanol = 0.81 g/mL × 6.2 mL = 5.022g
Moles of 1-butanol = 5.022g / 74.12 g/mol = 0.0677 mol
Theoretical yield = (0.0677 mol ×137.02 g/mol) / 1 mol of 1-bromobutane = 9.28g
Percent yield
Empty sample bottle= 14.97 g
Sample bottle+ product= 15.48 g
Mass of the product= 15.48g – 14.97g= 0.51g
Percent yield= (Actual yield/ theoretical yield) × 100
(0.51g/9.28g) × 100= 5.49 %
Synopsis of the Results
Analysis of the results shows that the empirical yield and theoretical yield of 1-bromobutane were quite different. The empirical yield of 1-bromobutane was 0.51g while the theoretical yield of 1-bromobutane was 9.28g. Comparative analysis of the yield shows that the 1-bromobutane yield of 0.51g represents 5.49% of the theoretical yield.
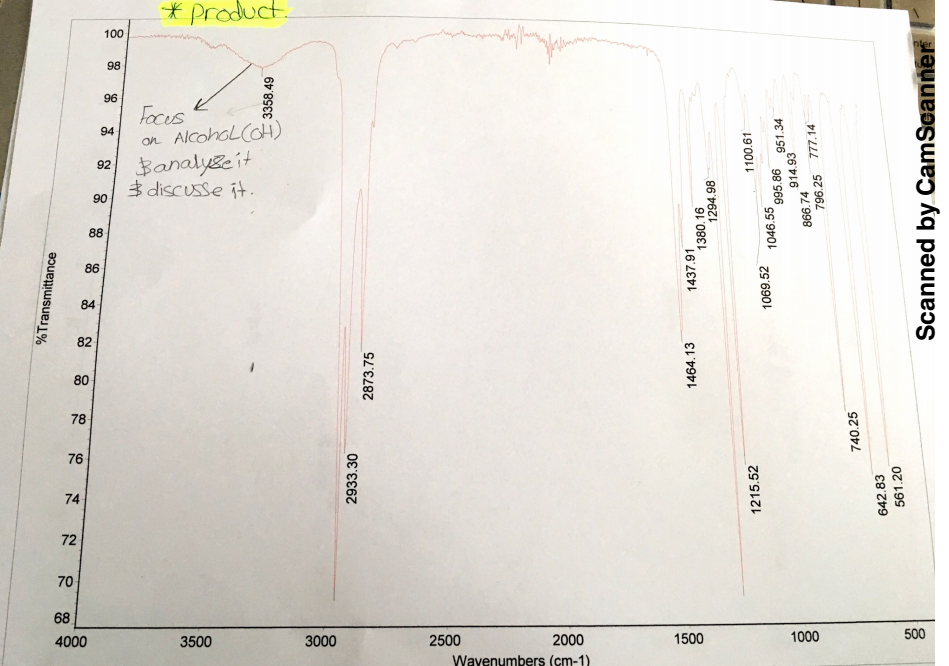
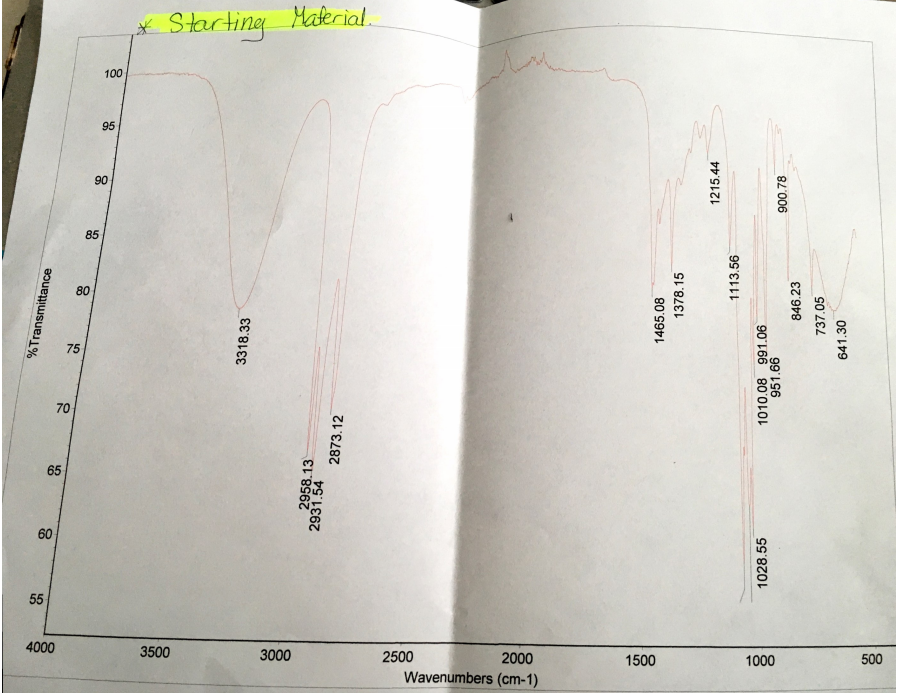
Evidently, several factors in the experiment contributed to the little yield of 1-bromobutane. One factor is that there was a little yield of 1-bromobutane because the substitution reaction did not convert most molecules of 1-bromobutanol into 1-bromobutane. The IR spectra before and after the reaction indicate that there are apparent changes in the O-H stretch between 3200cm-1 and 3500cm-1 wavenumbers. The IR spectrum for the O-H stretch before the reaction is broad and peaks at 3318.33cm-1 with a transmittance of about 80%, which indicates the presence of 1-butanol in the reactants.
Comparatively, the IR spectrum of the O-H stretch after the reaction is moderate and peaks at 3358.49cm-1 with a transmittance of about 98%. Hence, the stretch and transmittance that is apparent after the reaction indicate that some 1-butanol did not react with hydrogen bromide. Given that the experiment involved the heating of reactants, some 1-butanol molecules could have evaporated.
Moreover, 1-bromobutanol formed during the reaction might have leaked through the distillation apparatus because it is a very volatile compound. As the experiment involved washing with de-ionized water and sodium bicarbonate, some of 1-bromobutane might have remained with washes of the aqueous layers.
Discussion
The experiment demonstrated how substitution reactions of alcohols occur. A substitution reaction is a chemical reaction in which one functional group replaces another functional group resulting in a single displacement reaction. A substitution reaction can be either nucleophilic substitution or electrophilic substitution depending on the nature of the attacking functional group. When the attacking functional group has lone pairs of electrons and requires a cation to stabilize, then it is a nucleophile.
In nucleophilic substitution, a stronger nucleophile attacks cation and displaces a weaker nucleophile resulting in a displacement reaction. In contrast, when the attacking functional group has a positive charge and requires an anion to stabilize, then it is an electrophile. In electrophilic substitution, a stronger electrophile displaces a weaker electrophile resulting in a displacement reaction. Therefore, the nucleophilic or electrophilic strengths of functional groups determine their ability to displace other functional groups in diverse compounds.
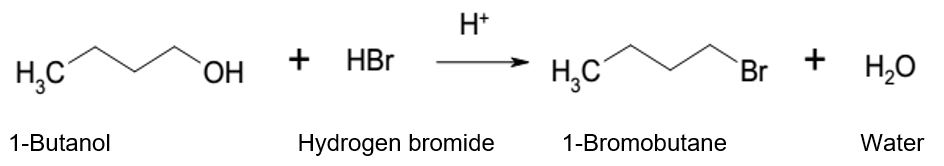
Specifically, the experiment demonstrated the nucleophilic substitution that leads to the formation of 1-bromobutane from 1-butanol. Alcohols are organic compounds that can undergo substitution reactions when they encounter nucleophiles in an acidic environment. 1-butanol is 4-carbon alcohol, and thus, the experiment used it to represent primary alcohols in the substitution reaction. The reactants for the nucleophilic substation are 1-butanol and hydrogen bromide in the presence of sulfuric acid.
The functional group of 1-butanol is hydroxyl (-OH) while the functional group of the hydrogen bromide is bromide ion (Br–). In the reaction, hydrogen ion (H+) from sulfuric acid protonates -OH group leading to the formation of a stable leaving group (-OH2). Given that Br– is a strong nucleophile, it attacks the protonated 1-butanol from the opposite side of -OH2– and displaces it as a water molecule. The nucleophilic substitution follows SN2 substitution because 1-butanol is a primary alcohol that forms unstable primary carbonation. In summary, the mechanism for the formation of 1-bromobutane from the reaction of 1-butanol and hydrogen bromide is a one-step reaction because there is no formation of a carbocation intermediate for nucleophilic substitution to occur.
In setting up the apparatus for the experiment, the gas trap was connected to the end of the vacuum hose. The purpose of the gas trap was to ensure that harmful gases do not escape into the air but dissolve in water. The heating of the mixture of 1-butanol, sulfuric acid, and hydrogen bromide produces a mixture of harmful gases that require containment to avoid environmental pollution. The design of the gas trap ensures that aspirated gases have sufficient surface area of water for them to dissolve when exposed or bubbled through the water. Hence, the gas trap in this experiment ensured that emitted gases do not contain vapor of sulfuric acid, hydrogen bromide, and 1-butanol.
After the reagents, 1-butanol, sulfuric acid, and hydrogen bromide were mixed in a round bottom flask, they were heated for 45 minutes under reflux. Heating under reflux is a form of heating that prevents reagents from escaping in the form of vapor when heated. It is applicable in heating volatile reagents that have low boiling points yet require high temperatures for them to react and give a required product. The apparatus for heating under reflux is a round bottom flask with reagents and a condenser with closed ends so that the condensed solution flows back into the boiling flask. Thus, heating under reflux prevents the loss of reagents through vapor and speeds the rate of reaction.
After heating the reagents under reflux for 45 minutes, the apparatus was converted into a simple distillation apparatus. The distillation process was done to separate the organic product, which is 1-bromobutane, from the mixture of organic and inorganic products. The distillation process was allowed to occur until the temperature reached 100°C because 1-bromobutane has a boiling point of 100°C. If distillation occurs at temperatures above 100°C, other compounds such as 1-butanol would distill, and thus, contaminate 1-bromobutane. Thus, the distillation process should stop at 100°C for the distillate to contain 1-bromobutane as the only organic product.
Organic and inorganic layers are two layers that the distillate contains. The upper layer is the organic layer while the lower layer is the inorganic layer. The product, 1-bromobutane, is in the upper layer for it is less dense than the aqueous layer. The organic layer comprises 1-bromobutane only, which has a density of 1.276g/mL. The aqueous layer is dense because it contains inorganic salts, sulfuric acid, and 1-butanol all of which are remnants of the reactants in the round bottom flask. The organic layer was washed with de-ionized water to eliminate acid residues. The organic layer was also washed with sodium bicarbonate to naturalize acids and create a basic aqueous solution, which enhances the separation of the organic layer and the inorganic layer. Furthermore, the organic layer was washed with de-ionized water to eliminate sodium bicarbonate. The drying agent, anhydrous sodium sulfate, absorbed water molecules from 1-bromobutane, and thus, made it dry.
What was accomplished in the experiment was the preparation of 1-bromobutane from the reaction of 1-butanol and hydrogen bromide in the presence of sulfuric acid. The analysis of the results indicates that there is a significant difference between empirical yield and theoretical yield of 1-bromobutane. The theoretical yield of 1-bromobutane is 9.28g whereas the empirical yield of 1-bromobutane is 0.51g. A comparison of the yields of 1-bromobutane indicates that the empirical yield of 0.51g constitutes 5.49% of the theoretical yield. The little yield of 1-bromobutane depicts that some errors contributed to such yields. The first possible source of error is that not all molecules of 1-butanol reacted to form 1-bromobutanol. The second possible source of error is the evaporation of 1-butanol during the heating process. The third possible source of error is that washing with de-ionized water and sodium bicarbonate removed some 1-butanol from the organic layer.
IR plays a central role in the analysis of results since it assesses the existence of 1-butanol before and after the reaction. Analysis of the generated IR spectra indicates that there are apparent changes in the O-H stretch between 3200cm-1 and 3500cm-1 wavenumbers before and after the reaction. Before the reaction, the IR spectrum for the O-H stretch is broad and peaks at 3318.33cm-1 wavenumber, which means that 1-butanol was present in the reactants. Moreover, the O-H stretch is unique because it has a transmittance of about 80% (See spectrum 1 in Appendix A). After the reaction, the IR spectrum of O-H stretch is moderate and peaks at 3358.49cm-1 wavenumber with a transmittance of about 98% (See IR spectrum 2 in Appendix B).
Comparison of the IR spectra before and after reaction shows that some 1-butanol did not react with hydrogen bromide, and thus, explains the occurrence of the little yield of 1-bromobutane. The theoretical yield assumes that all molecules of 1-butanol reacted with hydrogen bromide to give a 100% yield. However, the existence of errors, as confirmed by the IR spectrum, explains the occurrence of the little yield.
References
Chemical Book: 1-bromobutane 2008. Web.
Chemical Book: 1-butanol 2008. Web.
Chemical Book: Hydrogen Bromide 2008. Web.
Chemical Book: Sodium bicarbonate 2008. Web.
Chemical Book: Sodium sulfate 2008. Web.
Chemical Book: Sulfuric acid 2008. Web.
Questions
- Question 1
In the leaving group study, 2-bromo-2-methylpropane reacted faster than 2-chloro-2-methylpropane because it took a shorter time, which is 53 seconds for the color change to occur. - Question 2
Bromide ion is a better leaving group than chloride ion. The reason is that bromide ion more electronegative than chloride ion, and thus, it does not form a strong bond with carbon. - Question 3
2-bromo-2-methylpropane reacted faster than 2-bromopropane because it took 47 seconds for color change to occur. - Question 4
The above answer does not agree with the theory of the stability the intermediate carbocation. Water molecules can easily attack secondary and tertiary carbocation than primary carbocations, which contrasts the reactivity of alkyl halides with other nucleophiles. - Question 5
The solution of 40% isopropyl alcohol in water is a more polar solution than the solution of 60% isopropyl alcohol in water. Since water is a polar solvent, it means that a solution with a lot of water is more polar solution than the solution with a little water. - Question 6
My data do support that SN1 reactions proceed faster in a more polar solvent. The explanation is that the solution of propanol containing 60%, 50%, and 40% of water took 25 seconds, 47 seconds, and 101 seconds respectively for color change to occur.
Appendices
Appendix A: Spectrum
Appendix B: Spectrum 2