The Objective
The experiment aims to illustrate the way substitution reactions of alcohols transpire. In particular, the experiment aims to illustrate the way 1-butanol undergoes nucleophilic substitution reaction resulting in the formation of 1-bromobutane.
Synthetic Equation for the Reaction

1-Butanol Hydrogen bromide 1-Bromobutane Water
Physical Properties of Reactants and Products
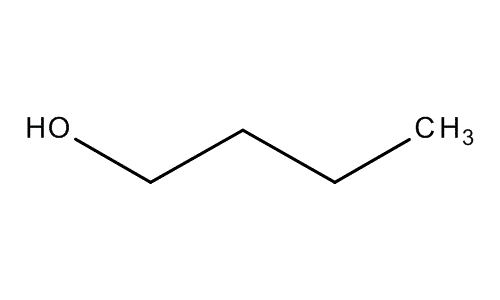
Molecular structure 1-Butanol
Physical state: Colorless liquid
Melting point: -89ºC
Boiling point: 117.6ºC
Molecular formula: C4H10O
Molecular weight: 74.12g/mol
Density: 810g/L
Water solubility at 20ºC: 80g/L
Hazards: Toxic, harmful, and highly flammable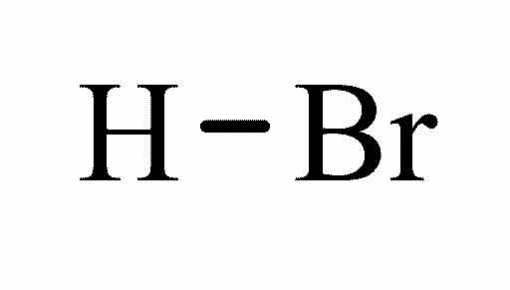
Molecular structure Hydrogen Bromide
Physical state: Colorless liquid
Melting point: -87ºC
Boiling point: -67ºC
Molecular formula: HBr
Molecular weight: 80.91g/mol
Density: 1490g/L
Water solubility at 20ºC: Miscible
Hazards: Irritant and corrosive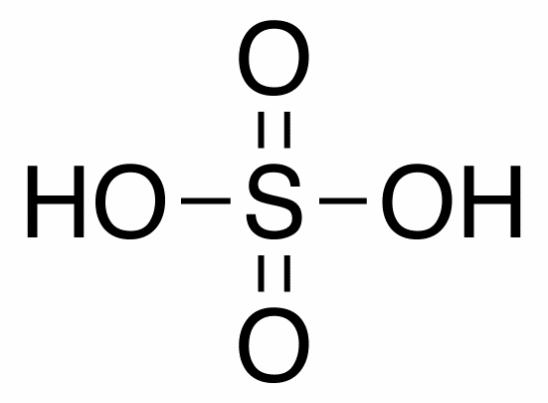
Molecular structure Sulfuric Acid
Physical state: Colorless oily liquid
Melting point: 10ºC
Boiling point: 290ºC
Molecular formula: H2SO4
Molecular weight: 98.08g/mol
Density: 1840g/L
Water solubility at 20ºC: Miscible
Hazards: Corrosive, highly flammable, toxic, and irritant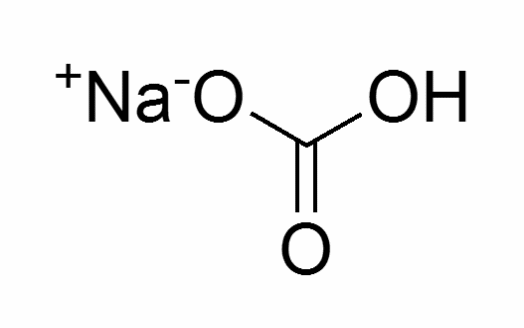
Molecular structure Sodium Bicarbonate
Physical state:White powder
Melting point: 300ºC
Boiling point: 851ºC
Molecular formula: NaHCO3
Molecular weight: 84.01g/mol
Density: 2160g/L
Water solubility at 20ºC: 90g/L
Hazards: Toxic and irritant
Molecular structure Sodium Sulfate
Physical state: White crystals or powder
Melting point: 884ºC
Boiling point: 1700ºC
Molecular formula: Na2SO4
Molecular weight: 142.04g/mol
Density: 2680g/L
Water solubility at 20ºC: 18mg/L
Hazards: Irritant
Molecular structure 1-Bromobutane
Physical state: Colorless-yellow liquid
Melting point: -112ºC
Boiling point: 100-101ºC
Molecular formula: C4H9Br
Molecular weight: 137.02g/mol
Density: 1276g/L
Water solubility at 20ºC: 0.608g/L
Hazards: Irritant, highly flammable, and harmful to environment
Procedure
The experiment was started by measuring 6.2ml of 1-butanol and transferring into a round bottom flask of 100ml. Subsequently, 10 ml of 48% hydrogen bromide (HBr) was measured and added to the round bottom flask with 6.2ml of 1-butanol while shaking. Similarly, 4ml of concentrated sulfuric acid (H2SO4) was measured and added carefully to the reactants in the round bottom flask while swirling and cooling in the ice bath. Afterward, a pair of boiling chips was added to the reactants in the flask, and a reflux condenser, water hoses, and vacuum tube were set up. The bottom end of the reflux condenser was connected to the flask, and the top end was joined to a vacuum tube while water hoses were joined at the inlet and outlet parts. The aspirator was connected to the end of the vacuum tube via the gas trap, and it was switched on to begin aspiration process. While the aspirator was on, a finger was put on the end of the vacuum tube to sense the suction pressure and proof that it was functioning well. When the experiment setup was confirmed working, and the reacting contents of the flask were heated at reflux for 45 minutes.
At the expiry of 45 minutes of heating, the reacting contents of the flask were cooled in the ice bath. Next, 10ml of deionized water was measured and added gradually to the cooled contents of the flask through the condenser. An additional pair of boiling chips was added to the cooled contents of the flask. The experiment setup was converted into a simple distillation system by disconnecting aspirator. The distillation process was performed, and a 25ml round bottom flask was immersed in the ice bath to condense and collect the distillate. When the temperature of the vapor attained 100ºC, the distillation process was halted.
The aqueous layer was removed from the distillate using transfer pipette and put in a different flask. The remaining organic layer was washed with 5ml of deionized water, and the aqueous layer was removed and added to the aqueous layer using transfer pipette. Subsequently, 5ml of 5% sodium bicarbonate was added to the organic layer, and the aqueous layer was removed and added to the aqueous layers in the flask collected previously. The organic layer was finally washed with another 5ml of deionized water, and the aqueous layer was obtained and added to the aqueous layers in the flask collected previously. Anhydrous sodium sulfate was added to the washed organic layer to dry. Once the organic layer dried, it was put into a pre-weighed sample bottle and capped as the product of the experiment. Ultimately, the product was weighed, an infrared spectrum assessed, and percent yield determined.
Results
Theoretical and Percent Yields of 1-Butanol
Theoretical yield
Mass of 1-butanol = volume × density = 6.2ml × 0.81 g/ml = 5.022g
Moles of 1-butanol = mass ÷ molecular mass = 5.022g ÷ 74.12 g/mol = 0.0678 moles
Theoretical yield of 1-bromobutane = molecular mass × moles = 137.02g × 0.0678 = 9.29g
Percent yield
- Empty sample bottle + cap = 25.21g
- Sample bottle + cap + product = 31.67g
- Mass of the product = (a – b) = 31.67g – 25.21g= 6.46g
Calculation of percent yield of 1-bromobutane = (Actual yield/ theoretical yield) × 100
= (6.46g/9.29g) × 100= 69.54%
Synopsis of the Results
The results indicate that the theoretical yield of 1-bromobutane was more than the empirical yield. Specifically, the theoretical yield of 1-bromobutane was 9.29g whereas the empirical yield was 6.46g. Calculation of the percent yield shows that empirical yield of 1-bromobutane constitutes 69.54% of the theoretical yield. The percent yield was not 100% because numerous factors in the experiment reduced the efficiency of the substitution reaction and other experimental processes. One possible factor that caused incomplete percent yield is that the substitution reaction did not convert all molecules of 1-butanol into 1-bromobutane. Another possible factor is that some 1-butanol molecules might have evaporated leading to their loss. The loss of 1-bromobutane through washing with deionized water and sodium bicarbonate could have resulted in the loss of the product. Furthermore, as 1-bromobutanol is a highly volatile compound, its loss could have occurred through leakage during distillation.
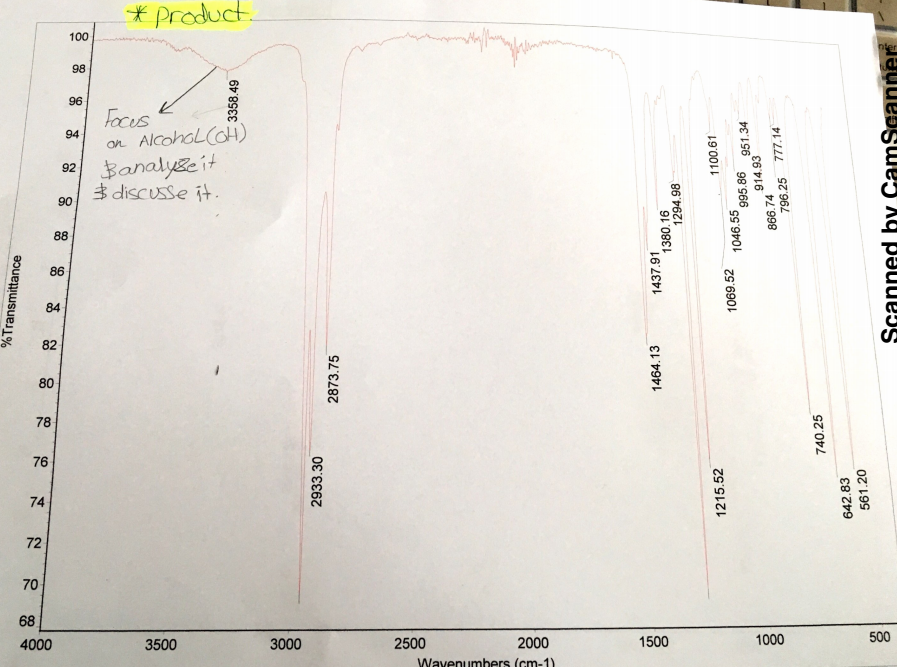
The analysis of the IR spectra before and after the reaction shows that there are some shifts in stretches. The O-H stretch at the range of 3200cm-1 to 3500cm-1wavenumbers has a noticeable shift. Before the reaction, the O-H stretch was very broad with a peak at 3318.33cm-1 and approximately 80% transmittance. The presence of this O-H stretch indicated that 1-butanol is in the reactants. After the reaction, the O-H stretch was moderately broad with a peak at 3358.49cm-1 and approximately 98% transmittance. In this view, the existence of O-H stretch and transmittance after the reaction showed that hydrogen bromide did not react with all 1-butanol. Moreover, the washing of organic phase with deionized water and sodium bicarbonate might have failed to remove all molecules of 1-butanol resulting in the detection of O-H stretch.
Discussion
The experiment effectively illustrated the way substitution reactions of alcohols happened and compared the theoretical and empirical yields of 1-bromobutane. Essentially, a substitution reaction is a form of single displacement reaction characterized by the replacement of a functional group by another one. Depending on the nature of the reacting functional groups, a substitution reaction can occur as an electrophilic substitution or a nucleophilic substitution. An electrophilic substitution occurs when an attacking functional group becomes an electrophile by losing electrons and gaining a positive charge, which seeks an anion to neutralize it. On the contrary, a nucleophilic substitution occurs when an attacking functional group becomes a nucleophile by gaining electrons and gaining a negative charge, which seeks cation to neutralize it. Fundamentally, the strength of an electrophile or nucleophile determines its ability to react by displacing another electrophile or nucleophile. In this case, a stronger nucleophile displaced a weaker nucleophile resulting in a displacement reaction.
The experiment effectively illustrated the way nucleophilic substitution resulted in the conversion of 1-butanol to 1-bromobutane. As alcohols are organic compounds with –OH as functional groups, they undergo substitution reaction in an acidic environment when they come across nucleophiles. The experiment used 1-butanol, which is an alcohol with four carbons, to represent other primary alcohols that undergo nucleophilic substitution reaction. In the experiment, 1-butanol and hydrogen bromide are the reactants for the nucleophilic substitution reaction, which reacts in an acidic environment created by sulfuric acid. The functional groups of 1-butanol and hydrogen bromide are hydroxide ion (-OH) and bromide ion (Br–) respectively.
As the mechanism of the nucleophilic substitution, hydrogen ions (H+) generated by sulfuric acid trigger reaction by protonating -OH group resulting in the formation of -OH2 group, which is a more stable leaving group than -OH group. Since Br– is an anion, it seeks cations and acts as a strong nucleophile that attacks the protonated -OH 2 group of 1-butanol and displaces it as water since it is a good leaving group. The mechanism of nucleophilic substitution reaction goes after SN2 substitution as it involves the formation of unstable primary carbonation from 1-butanol. Fundamentally, the formation of the unstable primary carbocation means that the mechanism for conversion of 1-butanol to 1-bromobutane is a single step because it lacks the formation of carbocation intermediate, which is essential in SN1 nucleophilic substitution mechanism.
In the experiment, the setup ensured that the gas trap was connected to one end of the vacuum tube. The function of the gap trap was to dissolve harmful gasses in water and prevent their escape into the air. The harmful gases emanate from the heating of reaction mixture comprising 1-butanol, hydrogen bromide, and sulfuric acid, which needs containment to prevent them from escaping and causing environmental pollution. The gas trap was designed to increase the surface area of water to absorb harmful gasses effectively. Therefore, the gas trap prevented the vapor of hydrogen, 1-butanol, and sulfuric acid from escaping and polluting the environment.
The reacting contents comprising 1-butanol, hydrogen bromide, and sulfuric acid were mixed and heated at reflux for 45 minutes. Essentially, heating at reflux is a heating mechanism that hinders heated substances from escaping as vapor. Normally, heating at reflux applies in heating volatile substances because they have low boiling points, but they need high temperatures to react and generate the specific product. The apparatus for heating at reflux encompassed the round bottom flask containing reacting contents and the condenser connected to the flask with its top end closed. Thus, the role heating at reflux was to not only contain the reactants and prevent them from escaping in the form of vapor but also increase the rate of reaction.
After heating the reacting contents at reflux for 45 minutes, the reflux apparatus was transformed into a simple distillation apparatus by removing aspirator. The purpose of the simple distillation process was to isolate 1-bromobutane, which is the organic product, from the mixture of reacting comprising organic and inorganic products. The distillation process was restricted to 100ºC because the boiling point of 1-bromobutane is 100ºC. The restriction of 100ºC is critical because temperatures that exceed would result in the distillation of 1-butanol and cause contamination of the product. Therefore, to obtain1-bromobutane as the pure product, the distillation should be restricted to100ºC.
The collected distillate contained two layers of organic and inorganic phases. The organic phased formed the upper layer whereas the inorganic phase formed the lower layer. As 1-bromobutane is an organic substance, it formed the upper layer because it has a lower density than the aqueous layer. Essentially, the organic phase has 1-bromobutane as the only substance, and it has a density of 1276g/L, which is denser than water. The aqueous layer is denser than water for it is a mixture of sulfuric acid, inorganic salts, and 1-butanol, which are residues of the reactants in the flask. Deionized water was used to wash the organic phase to remove acid residues. Sodium bicarbonate was also used to wash the organic phase to neutralize acids. Moreover, sodium bicarbonate improved the separation of inorganic phase and organic phase by creating an alkaline environment. In the final washing procedure, deionized water was used to wash the organic phase to remove sodium bicarbonate. In drying 1-bromobutane, anhydrous sodium sulfate was used as a drying agent because it can absorb water molecules from solids.
The experiment was successful because it managed to synthesize 1-bromobutane from the reaction of hydrogen bromide and 1-butanol in the acid conditions created by sulfuric acid. Comparative analysis of the results shows that theoretical yield of 1-bromobutane was significantly higher than the empirical yield. Evidently, the theoretical yield of 1-bromobutane was 9.29g while the empirical yield was 6.46g. The analysis of the percent yield reveals that empirical yield of 1-bromobutane forms 69.54% of the theoretical yield. The partial yield of 1-bromobutane shows that there are some possible sources of errors. A plausible source of error is that the incomplete reaction of 1-butanol molecules to form 1-bromobutane. The loss of 1-butanol and 1-bromobutane during heating and distillation is another plausible source of error that contributed to partial yield. As deionized water and sodium bicarbonate was used to wash the organic phase, it is plausible that these solutions washed away some 1-bromobutane.
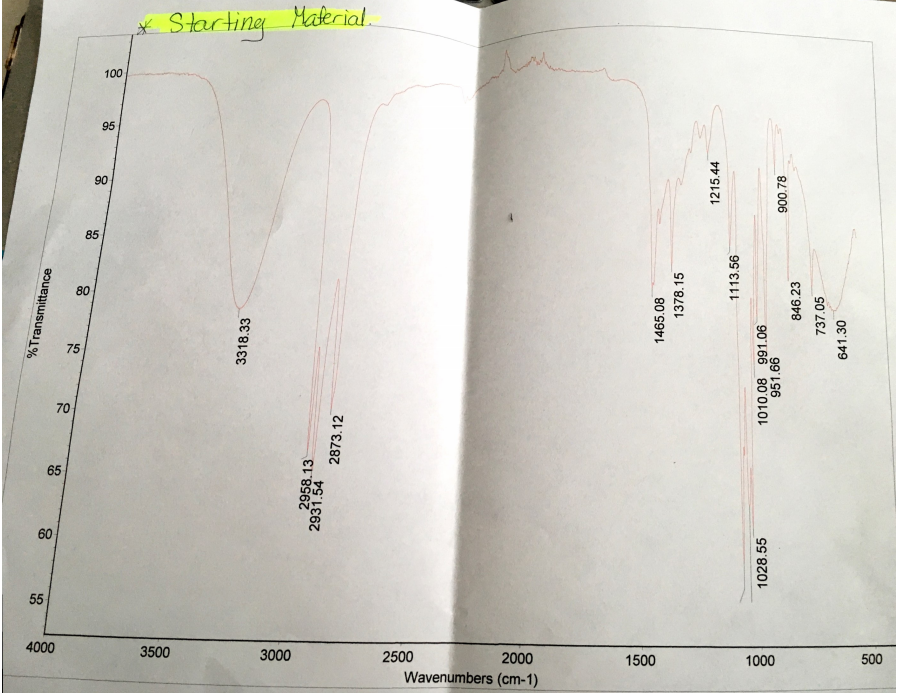
In this experiment, infrared is essential because it evaluates the presence of functional groups specific to reactants and products. The analysis of infrared spectra shows that there were changes before and after the reaction. The change reveals that the nucleophilic substitutions reaction took place. Specifically, the analysis of infrared spectra shows that there were significant shifts in the O-H stretch before and after the reaction at the range of 3200cm-1 to 3500cm-1. Before the reaction occurred, the O-H stretch in the IR spectra was very broad with a peak at 3318.33cm-1 and transmittance of approximately 80%, which indicates that the reactants contained 1-butanol and unique transmittance (Figure 1). After the occurrence of the reaction, O-H stretch in the IR spectra was moderately broad with a peak 3358.49cm-1 and transmittance of approximately 98% (Figure 2). Comparative analysis of the IR spectra indicates that residues of 1-butanol existed in products. The existence of 1-butanol in the product means some molecules did not react with hydrogen bromide resulting in the partial yield of 1-bromobutane.
References
1-bromobutane. ChemicalBook.2016. Web.
1-butanol. ChemicalBook, 2016. Web.
Hydrogen Bromide. ChemicalBook.2016. Web.
Sodium Bicarbonate. ChemicalBook. 2016. Web.
Sodium Sulfate. ChemicalBook.2016. Web.
Sulfuric Acid. ChemicalBook. 2016. Web.