Background
The phenomenon of the social anxiety disorder, also known as the social phobia, is typically interpreted as one of the numerous social communication disorders. In addition, according to the Diagnosis and statistical manual of mental disorders (5th ed.) (American Psychiatric Association, 2013), the subject matter is easily confused with some of the social communication disorders (Sureka, Desai, & Gupta, 2013). While admittedly having a range of characteristics in common, the above concepts are rarely interrelated. Therefore, the effects of the subject matter should be evaluated on their own merits as opposed to driving parallels with the social-communication-related issues (Sinacola, 2015; Yeun & Han, 2016).
Primary Objective
The efficacy of the intranasal Paroxetine as the SSRI tool (Hoang, Ha, & Quy, 2016) that may supposedly help address the issues related to the further development of the social anxiety disorder (SAD). The study is aimed, therefore, at proving that the use of the specified medicine allows for managing SAD efficiently as opposed to the use of placebo (Doobbs, 2013).
Secondary Objective
The research also seeks to locate the possible side effects of using the given medicine.
Design and Methodology
Design
Since the study focuses on the identification of qualitative relationships between the key variables, the adoption of a qualitative design seems a sensible approach to adopt. For example, the study may be conducted as a participant observation (Clarke, 2015).
However, one must also bear in mind that the research seeks to quantify the outcomes as well (Phillips et al., 2015). For instance, the location of the numerical correlation between the use of the identified types of medicine and the subsequent identification of the outcomes can be viewed as crucial to the assessment of the drug efficacy (Bryman, 2015).
Participants
In order to carry out the study, one will have to include two types of participants. The information necessary for the qualitative analysis will be gathered from the patients of the local healthcare facilities. Particularly, approximately 150 people will be recruited to participate in the study and respond to the questionnaire designed for a quantitative study (Bandelow et al., 2014).
As far as the qualitative element of the research is concerned, observations will be carried out in the experimental group (i.e., the participants that will be provided with Paroxetine) and the control group (i.e., the members of the study that will be given a daily amount of placebo). The changes in the patients’ behavioral patterns will be identified and evaluated carefully so that the dynamics of anxiety in both groups could be evaluated and compared consequently (Book et al., 2013; Parsons, 2015).
Inclusion criteria
The patients will be included based on the characteristics such as their diagnosis (particularly, the patients must have a social anxiety disorder), their age (it is preferable that people aged 16-20 should partake in the process), and any specific drug idiosyncrasy should be considered a candidate for the experiment.
Exclusion criteria
To participate in the study, one must not have any issues outside of the identified one and be mentally stable (Anney, 2014; Tomita et al., 2014).
Ethics
The research will be carried out base on the primary principles of confidentiality and consent. Therefore, the participants will be provided with complete anonymity, to the point, where their real names will be replaced by “Participant 1,” “Participant 2,” etc. In addition, patients under 18 will not be allowed to participate in the study unless they have the permit of their parents or guardians (Enck, Klosterhalfen, Weimer, Horing, & Zipfel, 2011).
Finally, every single member of the experiment will be provided with the informed consent form that will detail the objectives of the research, the information used in the experiment, and other essential characteristics thereof (Hoang, Ha, & Quy, 2016). The applicants in their turn, will be suggested to sign the form if they agree to take part in the study or to decline it if they do not. The above measures will be taken not to avoid possible legal repercussions but to ensure the safety and security of the people involved in the study (Roest et al., 2015; Upadhyaya et al., 2013).
Methodology
Time frame
It is expected that the research will be taking place for at leats two months. The choice of the specified time frame can be justified by the fact that the changes, which the medication is supposedly going to have on the patients, will only become easily identifiable after two weeks. Until the changes in the patients’ behavioral patterns can be deemed as permanent, the veracity of the research outcomes will remain dubious.
Sample size calculation
In order to carry out the calculations of the sample size, one should consider the formula that Charan and Biswas (2013) view as the primary means of extracting the information regarding the sample size. According to the authors of the study mentioned above, the sample size population can be estimated using the formula below:
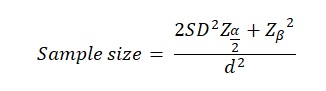
where Z is the Type I error. Given the formula above, the sample size of the participants, who will contribute to the analysis of the study and the further production of the results will equal:
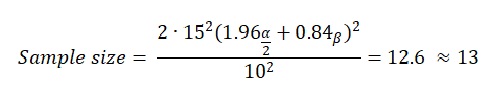
The sample size of the population, therefore, is 13.
Statistical analysis
Although carrying out vast qualitative research is crucial to understanding the relationships between the variables in the study, it is still necessary to quantify the specified links so that the effects thereof could be assessed and, therefore, a proper measurement instrument could be incorporated into the further treatment process. Seeing that the paper is primarily aimed at assessing the effects of a single factor, i.e., the consumption of the medicine identified above, it will be reasonable to suggest that a one-way ANOVA analysis strategy should be used.
Expenditures
Since the project under analysis involves a plethora of opportunities for using the latest technological advances and demands that the facilities and treatment of the finest quality should be provided for the patients, it will be necessary to consider some of the most likely expenditures to be taken. As the table below shows, the project under analysis is going to be rather costly as far as the purchase of the necessary components thereof is concerned (Larson et al., 2015). It should be noted, though, that the adoption of a lean approach will serve as the primary tool for retrieving the necessary financial resources within a comparatively short amount of time. Specifically, the approach outlined above requires that the current strategy of resources allocation should be renewed (Levenson et al., 2014).
Rationale
Pharmacodynamics
N the course of the experiment, the participants will be exposed to the influence of Paroxetine and a placebo (Channareddy, 2013). Specifically, the m embers of Group A will be exposed to an increasing influence of the above drug. The dose will be increasing from 20 mg to 27 mg in the course of the two weeks that the experiment will last at the rate of a 0.5-mg change per day being the key rate (Kamaradova, Prasko, Sandoval, & Latalova, 2014).
Statistics
The quantitative study will demand to adopt descriptive statistics as the primary tool for extracting and analyzing data. It is recommended that the SPSS tools should be regarded as the primary means of data analysis. The reasons for choosing the above tool is that the software in question has been designed specifically or qualitative data analysis. However, one must admit that the use of Excel is also a possibility (Petersen & Aberg, 2014).
Strengths
The paper proposed has a range of strengths, the focus on the evidence-based approach being the primary one. As soon as the audience realizes that retrieving data is only possible after careful observations and the conclusions based solely on facts, a huge leap of science is expected. In addition, the adoption of the ANOVA tool as the means of carrying out a statistical analysis is the first step toward gaining academic credibility and delivering verified results. Therefore, premises for altering the current strategy of Paroxetine provision to the target denizens of the population may be altered (Roest et al., 2015).
Limitations
Naturally, the research has its limitations, the possibility for some of the participants to develop resistance towards Paroxetine being the primary one (Giménez et al., 2014). As a result of the above phenomenon, the objectivity of the research outcomes may be jeopardized. The specified limitation can be addressed by carrying out regular observations of the members of both groups so that the slightest changes in the pharmacokinetics. Therefore, there is a slight possibility that the veracity of the research results may be reduced significantly in case some of the patients develop resistance toward the identified medicine.
Reference List
American Psychiatric Association. (2013). Diagnosis and statistical manual of mental disorders (5th ed.). Washington, DC: APA.
Anney, V. N. (2014). Ensuring the quality of the findings of qualitative research: Looking at trustworthiness criteria. Journal of Emerging Trends in Educational Research and Policy Studies (JETERAPS), 5(2), 272-281.
Bandelow, B., Boerner, R. J., Kasper, S., Linden, M., Wittchen, H.-U., & Möller, H.-J. (2014). The Diagnosis and treatment of generalized anxiety disorder. Deutsches Ärzteblatt International, 110(17), 300-310.
Book, S. W., Thomas, S. E., Smith, J. P., Randall, P. K., Kushner, M. G., Bernstein, G…, & Randalla, C. L. (2013). Treating individuals with social anxiety disorder and at-risk drinking: Phasing in a brief alcohol intervention following Paroxetine. Journal of Anxiety Discord, 27(2), 252-258.
Bryman, A. (2015). Social research methods. Web.
Bystritsky, A., Khalsa, S. S., Cameron, M. E., & Schiffman, J. (2013). Current diagnosis and treatment of anxiety disorders. Pharmacology & Therapeutics, 38(1), 30-45.
Channareddy, L. R. (2013). Escitalopram reduces severity of depression and improves quality of life in patients with chronic obstructive pulmonary disease in an open label parallel group study. International Journal of Basic & Clinical Pharmacology, 5(2), 281-284.
Clarke, V. (2015). Review of the book ”Interpretative phenomenological analysis: Theory, method and research”. Psychology Learning & Teaching, 9(1). 57-56.
Dobbs, A. S. (2013). A little better than placebo is still better than nothing . Nature Medicine, 19(8), 962.
Enck, P., Klosterhalfen, S., Weimer, K., Horing, B., & Zipfel, S. (2011). The placebo response in clinical trials: More questions than answers. Philosophical Transactions of the Royal Society, 366(1572), 1889–1895.
Giménez, M., Ortiz, H., Soriano-Mas, C., López-Solà, M., Farré, M., Deus, J.,… & Merlo-Pichcorrespondence, E. (2014). Functional effects of chronic paroxetine versus placebo on the fear, stress and anxiety brain circuit in Social Anxiety Disorder: Initial validation of an imaging protocol for drug discovery. European Neuropsychopharmacology, 24(1), 105-116.
Goldin, P. R., Ziv, M., Jazaieri, H., Hahn, K., Heimberg, R., & Gross, J. J. (2013). Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs randomized clinical trial. JAMA Psychiatry, 70(10), 1048-1056. Web.
Hoang, T. P., Ha, T. N., & Quy, H. K. (2016). Factors affecting the service quality standards at the international airports when Viet Nam integrates TPP: A study at TAN Son Nhat Airport, Ho Chi Minh City, Vietnam. British Journal of Marketing Studies, 4(1), 43-52.
Kamaradova,D., Prasko, J., Sandoval, A., & Latalova, K. (2014). Therapeutic response to complex cognitive-behavioral and pharmacological treatment in patients with social phobia. Activitas Nervosa Superior Rediviva, 56(3–4), 91-99.
Larson, B. A., Bii, M., Henly-Thomas, S., McCoy, K., Sawe, F., Shaffer, D., & Rosen, S. (2013). ART treatment costs and retention in care in Kenya: a cohort study in three rural outpatient clinics. Journal of International AIDS Society, 16(18026), 15. Web.
Levenson, J. C., Troxel, W. M., Begley, A., Hall, M., Germain, A., Monk, T. H., & Buysse, D. J. (2014). A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. ICSM, 9(2), 124-153.
Parsons, T. D. (2015). Virtual reality exposure therapy for anxiety and specific phobias. Psychology and Human Behavior, 1(1), 288-2296. Web.
Petersen, J., & Aberg, N. (2014).Investigation of antecedents to performance in Swedish small and medium-sized companies. Web.
Phillips. K., Zai, G., King, N. A., Menard, W., Kennedy, J. L., &. Richter, M. A. (2015). A preliminary candidate gene study in body dysmorphic disorder. Journal of Obsessive-Compulsive and Related Disorders, 6(1), 72-76.
Roest, A. M., Jonge, P. d., Williams, C. D., Vries, Y. A. d.,. Schoevers, R. A., & Turner, E. H. (2015). Reporting bias in clinical trials investigating the efficacy of second-generation antidepressants in the treatment of anxiety disorders. JAMA Psychiatry, 72(5), 500-510. Web.
Sinacola, R. S. (2015). Pharmacologic management of anxiety spectrum disorders. Audio-Digest Psychology, 4(1), 1-3.
Sureka, P., Desai, N., & Gupta, D. K. (2013). A study of subsyndromal and syndromal psychiatric morbidity among male patients with alcohol dependence. ASEAN Journal of Psychiatry, 14(2), 146-156.
Tomita, T., Norio, Y.-F., Sato, Y., Nakagami, T., Tsuchimine, S., Kaneda, A., & Kanek, S. (2014). Sex differences in the prediction of the effectiveness of paroxetine for patients with major depressive disorder identified using a receiver operating characteristic curve analysis for early response. Neuropsychiatric Disease and Treatment, 10, 599–606. Web.
Upadhyaya, H., Adler, L. A., Casas, M., Kutzelnigg, A., Williams, D., Tanaka, Y., Arsenault, J., Escobar, R., & Allen, A. J. (2013). Baseline characteristics of European and non-European adult patients with attention deficit hyperactivity disorder participating in a placebo-controlled, randomized treatment study with atomoxetine. Child and Adolescent Psychiatry and Mental Health, 7(1), 14-22.Web.
Yeun, Y.-R., & Han, J.-W. (2016). Effect of nurses’ organizational culture, workplace bullying and work burnout on turnover intention. International Journal of Bio-Science and Bio-Technology, 8(1), 372-380. Web.