The purpose of the experiment
The purpose of the experiment is to synthesize an ester called methyl salicylate in the laboratory using the chemical process of Fischer esterification. The chemical process in this experiment entails reacting salicylic acid and methanol in presence of concentrated sulfuric acid, which catalyzes the reaction, and under reflux conditions to form methyl salicylate and water.
Synthetic Equation
Structures, Physical Properties, and Hazards of Reactants and Products
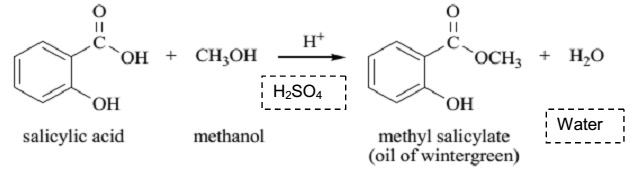
Salicylic Acid
Molecular structure:
Molar mass: 138.12 g/mol
Molecular formula: C7H6O3
Melting point: 158-161°C
Boiling point: 211°C
Density: 1.4 g/ml
Water solubility: 1.8 g/L
Hazards: Highly flammable and harmful
(“Chemical Book: Product Chemical Properties” par. 2)
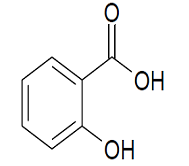
Methanol
Molecular structure:
Molar mass: 32.04 g/mol
Molecular formula: CH3OH
Melting point: -98°C
Boiling point: 65.4°C
Density: 0.791 g/ml
Water solubility: Soluble
Hazards: Highly flammable, toxic, and harmful
(“Chemical Book: Product Chemical Properties” par. 3)
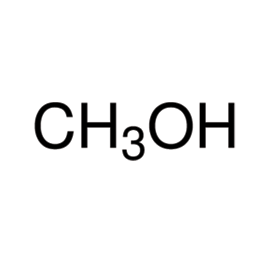
Sulfuric Acid
Molecular structure
Molar mass: 98.08 g/mol
Molecular formula: H2SO4
Melting point: 10°C
Boiling point: 290°C
Density: 1.84 g/ml
Water solubility: Soluble
Hazards: Highly flammable, corrosive, toxic, and harmful
(“Chemical Book: Product Chemical Properties” par. 2)
Methylene Chloride
Molecular structure:
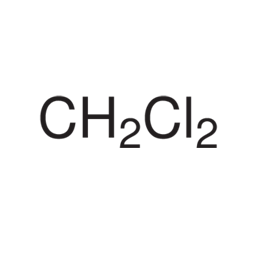
Molar mass: 85.94 g/mol
Molecular formula: CH2Cl2
Melting point: -97°C
Boiling point: 39.8-40°C
Density: 1.325 g/ml
Water solubility: Soluble
Hazards: Harmful
(“Chemical Book: Product Chemical Properties” par. 3)
Sodium Bicarbonate
Molecular structure:
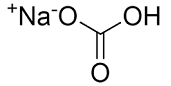
Molar mass: 84.01 g/mol
Molecular formula: NaHCO3
Melting point: 300°C
Boiling point: 851°C
Density: 2.16 g/ml
Water solubility: 0.09 g/ml
Hazards: Harmful and irritant
(“Chemical Book: Product Chemical Properties” par. 2)
Anhydrous Sodium Sulfate
Molecular structure:
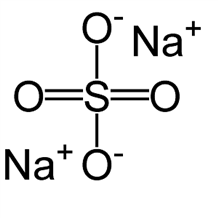
Molar mass: 142.01 g/mol
Molecular formula: Na2SO4
Melting point: 884°C
Boiling point: 1700°C
Density: 2.68 g/ml
Water solubility: 0.0189 g/ml
Hazards: Irritant.
(“Chemical Book: Product Chemical Properties” par. 4)
Methyl Salicylate
Molecular structure:
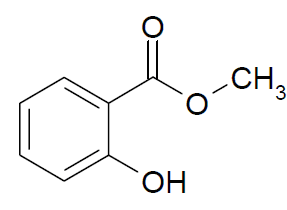
Molar mass: 152.15 g/mol
Molecular formula: H8H8O3
Melting point: -5°C
Boiling point: 219°C
Density: 1.174 g/ml
Water solubility: Soluble
Hazards: Harmful
(“Chemical Book: Product Chemical Properties” par. 3)
Water
Molecular structure:
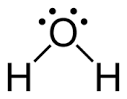
Molar mass: 18.02 g/mol
Molecular formula: H2O
Melting point: 0°C
Boiling point: 100°C
Density: 1 g/ml
Hazards: Corrosive
(“Chemical Book: Product Chemical Properties” par. 3)
Procedure
4.9 g of salicylic acid was weighed precisely and was then put in a 50 ml round-bottom flask. Consequently, exactly 12.5 ml of methanol was measured and added to the 50 ml flask with salicylic acid while swirling until all the solids dissolved. Cautiously, 5 ml of sulfuric acid was measured and added slowly to the contents in the 50 ml flask while swirling to dissolve white precipitate formed. Subsequently, a reflux condenser was fixed to the flask with the contents and then heated at reflux for 45 minutes. The reacting contents at reflux were swirled periodically as the oil layer was formed on top of the flask.
After 45 minutes of heating at reflux, the reaction mixture in the flask was cooled to room temperature. Then, 10 ml of ice water was taken and added to the cooling contents of the flask and subsequently transferred to a separatory funnel. The product of the reaction, which is oil of wintergreen, was extracted consecutively with two portions of 15 ml methylene chloride while shaking the contents gently. The two portions of methylene chloride extracts were combined into one and were washed first with 15 ml of water followed by 15 ml of 5% aqueous sodium bicarbonate solution. The organic layer was obtained from washing was dried over sodium sulfate and was decanted into a pre-weighed 100 ml round-bottom flask. The dried organic product was then evaporated using a rotary evaporator while taking great caution to prevent its evaporation with the solvent. The product formed was obtained, weighed, and the percent yield calculated. Moreover, IR and NMR were run to analyze the product formed.
Calculation of the Theoretical Yield and Actual Yield
Determination of the limiting reactant
The number of moles of salicylic acid used: 4.9g/138.12 g/mol= 0.035 moles
The mass of methanol used: 12.5 ml * 0.791 g/ml = 9.88 g
The number of moles of methanol used = 9.88 g/32.04 g/mol= 0.308 moles
The calculations show that salicylic acid is the limiting reactant.
Theoretical yield
As the reaction ratio of salicylic acid and methyl salicylate is 1:1, theoretically, it means that 0.035 moles of salicylic acid produces 0.035 moles of methyl salicylate.
The mass of methyl salicylate = 0.035*152.15 g =5.3 g
Actual yield
Empty round flask = 52.72 g
Round flask + product = 57.72 g
Product = 57.72 g – 52.72 g = 5.00 g
Percent yield
% Yield= Actual yield/Theoretical yield * 100
% Yield = 5.0 g/5.3 g *100= 94.33%
Synopsis of Results
The determination of the limiting reactant reveals that salicylic acid was limiting reactant because it had smaller amount moles than methanol. The calculations also reveal that the theoretical yield of 5.3 g while the actual yield is 5.00g. The calculations means that the percent yield of methyl salicylate is 94.33% of the theoretical yield. The percent yield is very high owing to the efficiency of the experimental setup in carrying out esterification reaction. The possible sources of errors that led to the loss of 5.67% of salicylate are partial reaction of methanol and salicylic acid, loss through aqueous phase during washing, and evaporation during reflux heating.
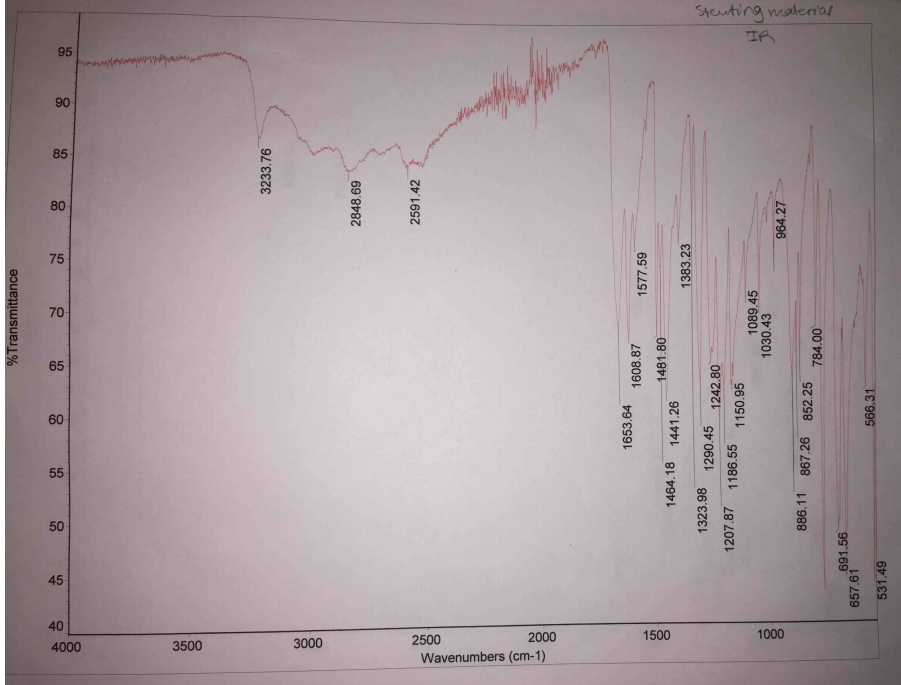
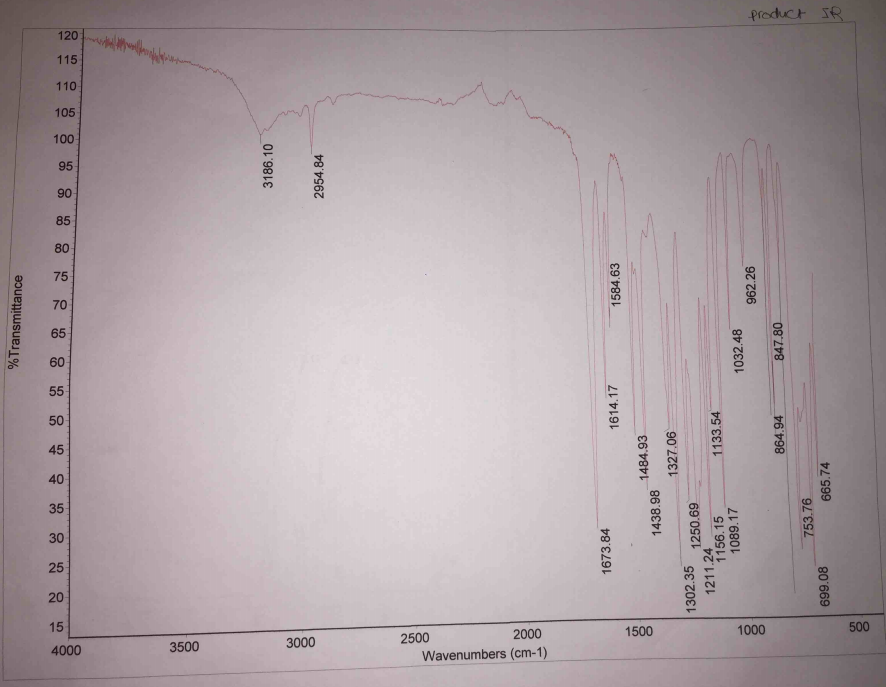
The IR spectra have landmark stretches that indicate the changes in the chemical nature of reacting material and products. Specifically, the IR spectra of the reacting material have O-H stretches at 2848.69 cm-1, 2591.42 cm-1, and 3233.76 cm-1 (Figure 2. In comparison with the IR spectra of the product, the IR spectra have O-H stretches at 3180.62 cm-1 and 2954.77, and C=O stretch at 1673.77 cm-1 (Figure 3).
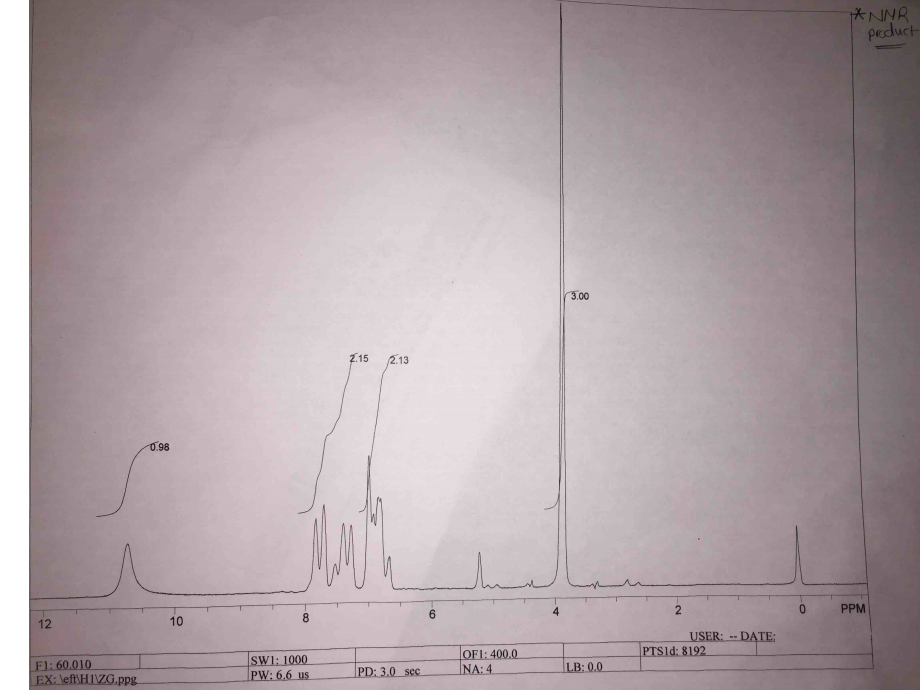
The NMR spectra also show that there are chemical shifts, which reflect the chemical shifts of methyl salicylate as shown by the four peaks, namely, 3.0, 2.13, 2.15, and 0.98 (Figure 4). The peak of 3.0 is singlet at 4 PPM, the peaks of 2.13 and 2.15 at 6-8 PPM form doublet, and the peak of 0.98 is a singlet at 11 PPM.
Discussion
The experiment, which aimed to synthesize methyl salicylate, was successful because the percent yield of the product was 94.33% of the theoretical yield. Fundamentally, the reaction between methanol and salicylic acid in the presence of concentrated sulfuric acid and heat constitute an esterification reaction. The esterification reaction is a form of condensation reaction because there is the formation of water molecules. The mechanism of action is dependent on the acidic conditions and heat. Concentrated sulfuric acid supply protons, which protonates the carbonyl group of salicylic acid and forms a primary carbocation. Subsequently, the lone pairs of electrons in the methanol attack the primary carbocation and create resonance structures. Eventually, the protonation of the –OH group of salicylic acid by the hydrogen tranfered from methanol creates a good leaving group as water and the formation ester.
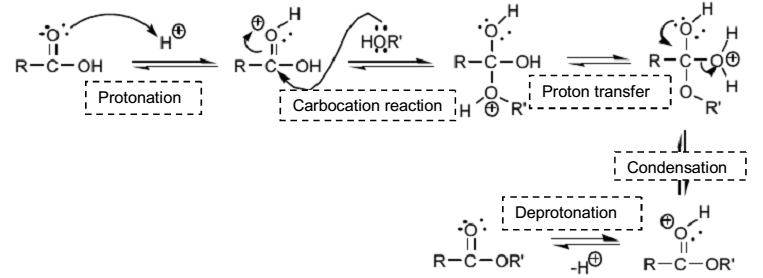
The purpose of the concentrated sulfuric acid in the experiment is to catalyze the reaction between salicylic acid and methanol. The reaction mechanism of esterification (Figure 5) shows that concentrated sulfuric acid offer protons, which protonate carbonyl group of salicylic acid and form a primary carbocation; hence, initiating the reaction. During the gradual addition of concentrated sulfuric acid, white precipitate was formed. The white precipitated formed was a polymer of salicylic acid. Acton states that salicylic acid polymerize when reacted with excess concentrated sulfuric acid (112). The following equation (Figure 6) shows the formation of the white precipitated.
The reacting contents in the flask were heated at reflux to increase the reaction rates and prevent the loss of solvent through evaporation. Reflux is heating system that allows the reacting contents to attain their boiling points and heated for a required period without their loss because they are in a closed system. In reflux system, the solvent evaporates and condense in cooler parts of the system where flowing water bring about the cooling effect, and eventually returns to the system for continued heating. After heating at reflux, the contents were cooled and added ice water to stabilize the product and prevent its hydrolysis. Palvia explains that methyl salicylate is a delicate product that is prone to decomposition when conditions favor backward reaction (97). Moreover, the addition of ice water provided an aqueous environment for the removal of solutes.
The extraction process of the product from the aqueous phase into organic phase involved double extraction using methylene chloride. Double small extraction is better than a single big extraction because of the extraction equilibrium state created. The efficiency of an extraction process is dependent on the number of equilibrium states created between organic and inorganic solutions (Brunner 59). The extraction and washing formed two layers of aqueous and organic components. Given that methyl salicylate (1.174 g/ml) is denser than water (1 g/ml), it formed the lower layer of contents in the flask.
The contents were washed with water to remove solutes from the solution and retain methyl salicylate. Sodium bicarbonate was subsequently added to the contents to neutralize acids. The reaction between sodium bicarbonate and acids generates carbon dioxide, which is evident by fizzing. The following is a neutralization reaction:-
NaHCO3–(aq) + H+ (aq) → Na (aq) + CO2 (g) + H2O (l)
In drying the product, the experiment used drying agent called anhydrous sodium sulfate. The drying agent is appropriate because it does not interfere with the state and chemical nature of the product. A molecule of anhydrous sodium sulfate can absorb ten molecules of water as indicated in the following equation.
Na2SO4(l) + 10H2O (I) → Na2SO4.10H2O (s)
To obtain the product, gravity filtration was used in filtering the organic phase. Gravity filtration is the simplest form of filtration because it relies on gravity in separating solvent from solids. Ultimately, the experiment used rotary vapor in removing solvent from the product. A rotary vapor uses a suction pressure in removing solvent in form of vapor during heating. To avoid the loss of product, the evaporation was stopped when the solvent was no longer in the flask.
The IR spectra of the reacting material and the products have different stretches, which indicate that there were changes in the nature of reacting materials. The reacting material has a broad O-H stretch at 2848.69 cm-1 and 2591.42 cm-1, which reflect that of carboxylic acid (salicylic acid). Additionally, the reacting material has an O-H stretch at 3233.76 cm-1, which reflects that of alcohol (methanol) as indicated in Figure 2. Analysis of the IR spectra of the product indicates that it has no broad O-H stretch of carboxylic acid at 2591.42 cm-1, hence, indicating that the product has no salicylic acid. However, it maintained O-H stretches at 3180.62 cm-1 and 2954.77 cm-1showing that there are traces of methanol for they were in excess. The IR spectra of the product have a landmark C=O stretch at 1673.77 cm-1, which reflect the presence of ester (methyl salicylate). Therefore, the changes in the stretches of the IR spectra indicate that there was synthesis of methyl salicylate when methanol and salicylic acid reacted.
The NMR analysis of the product shows that the chemical shifts reflect that of methyl salicylate. Notable peaks of the NMR spectra are 3.0, 2.13, 2.15, and 0.98. The major peak of 3.0 is visible at 4 PPM, and it represents three protons that are in the methyl group of methyl salicylate. At 6-8 PPM, there are two peaks (doublets), which represent the protons that are in the benzene ring of the methyl salicylate. The single peak of 0.98 at 11 PPM represents the proton in the –OH of methyl salicylate. Hence, the chemical shifts in NMR confirm that the product is methyl salicylate.
Calculations indicate that the percent yield of the product is 94.33% of the theoretical yield because of some errors that are inherent in the experimental procedure. One possible source of error is the partial reaction of methanol and salicylic acid leaving some of the reactants unconverted. Another possible source of error is the loss of the product during washing through the aqueous phase. As the reaction process involved heating at reflux, some of the product might have evaporated due to overheating and leakage of reflux system.
Works Cited
Acton, Ashton. Salicylic Acids: Advances in Research and Application. New York: Cengage Learning, 2013. Print.
Brunner, Gerd. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes. Darmstadt: Steinkopff, 2013. Print.
Chemical Book: Product Chemical Properties 2008. Web.
Palvia, Donald. Introduction to Organic Laboratory Techniques: A Small-Scale Approach. Belmont: Thomson Brooks/Cole, 2005. Print.