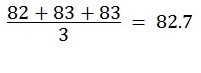Introduction
The boiling point denotes the temperature position where the liquid-vapor pressure establishes an equilibrium with the atmospheric pressure, thus necessitating the liquid to transform into the gaseous phase (boiling). The molecular configuration of compounds in the liquid state is relatively closely packed compared to particles in gaseous compounds. This molecular proximity permits them to interact via non-covalent bonds.
The interactions assist the binding of molecules in a definite volume and flow freely within the confined space. However, molecules of gaseous composites in a particular enclosed volume are sparsely distributed, thus having weaker non-covalent forces than liquids. Consequently, when the liquid is heated, the molecular interactions are broken by the energy, thus changing to the gaseous state. Conceptually, the transition is signified by the formation of vapor bubbles.
This experiment aims to provide acquaintance with the steps involved in physical properties evaluation and recognize unidentified samples using their boiling points. The test employs a temperature range to ascertain the boiling points of the given organic liquids. This temperature domain symbolizes the liquid-gas shift at 760 mmHg of pressure (Boiling Point, 2021). Nevertheless, several organic compounds may share similar boiling points despite the element being an intrinsic physical feature of a blend.
Experimental Procedure
A volume of about 5 ml of the liquid was inserted in a 10 ml test tube. A one-end wrapped capillary tube was immersed into the liquid with its unsealed end down. The test tube was tightly secured onto a thermometer using a rubber band. After that, the whole apparatus assembly was clamped to the ring stand. The assembly was then placed in a water bath with a provision of a standby oil bath for the specimen with boiling points exceeding 100 °C.
Gradual increment in temperature resulted in rapid effervescence from the end of the tube. The apparatus was heated for 10 seconds to ensure complete air expulsion from the capillary tube while the liquid vapors remained confined. The entire assembly was then maintained in the water bath throughout heat dispensation.
The observed liquid boiling point was read and recorded from the thermometer after the lapse of bubble formation. The boiling point experimental results were then compared with the literature values for ascertaining the technique’s efficiency. The above steps were then repeated with known liquids, and capillary tubes were used for every liquid. Prior to redoing the experiment, it was also ensured that the hot water bath cooled by 20°C lower than the reckoned boiling point.
Results and Discussion
Part I. Boiling Points of Known Compounds
Table 1. Experimental and literature boiling point ranges of known compounds
Part II. Boiling Point of an Unknown Sample
Table 2. Experimental boiling point range of unknown sample
Calculations
Table 3. Tabulation of boiling point range deviations
Table 4. Average boiling point for unknown sample
The experiment did not utilize the oil bath since none of the compounds exhibited a boiling point higher than 100°C. During heat removal, it was observed that bubbling was persistent for some time. The stoppage of any visible bubbles indicated the equilibrium between the atmospheric pressure and the liquid-vapor pressure. The rate of bubbles production decreased with the rise in liquid level in the capillary tube and slowing down of temperature.
From the first phase of determining boiling points of known compounds A, B, and C (t-butyl alcohol, isopropyl alcohol, and ethanol), the values were found to be 82°C, 83°C, and 78°C, respectively. The experimental data of these practice compounds deviated from the theoretical values by+1.0, -0.5, and +0.5. The errors would have been contributed by human errors emanating from practices such as the inability to precisely ascertain bubbling cessation and parallax error when recording the thermometer’s temperature readings.
Finally, the second part of the experiment gave an average boiling point temperature of 82.7°C. The comparison of this experimental value with the literature data for the provided known compounds was proportionate to that of t-butyl alcohol. The highest recorded deviation from the comparison of all the experimental results with the theoretical value was 1.0°C. The difference was below the stipulated maximum absolute variance of 2 °C to 3 °C; thus, the technique was efficient.
Conclusion
The experiment employed micro methods to demonstrate the boiling points of the provided organic compounds. The exercise offered a platform to familiarize me with the procedures for physical properties assessment for various compounds and the knowledge of identifying unknown solutions using the acknowledged values of recognized samples. Subsequently, the experiment was successful as the boiling points of t-butyl alcohol, isopropyl alcohol, and ethanol were found to be 82°C, 83°C, and 78°C. Further, the unknown liquid specimen was revealed to be t-butyl alcohol. The experiment’s efficiency would be improved by minimizing experimental errors by using a digital apparatus and undertaking the practical in a controlled environment.
Reference
Introductory Chemistry. (2021). Web.