Discovery and History of Glutamate
Glutamate, commonly termed monosodium l-glutamate (MSG), was discovered in 1908 by Professor Kikunae Ikeda. Foods have sweet, sour, salty, and bitter tastes but a new taste discovered, could not be classified into the four common tastes hence the name umami was born. Professor Ikeda did experiments to determine the cause of the umami taste using seaweed called kombu. After many trials, he found out the source of umami was glutamate and used it to craft a type of food seasoning (Sano, 2009). From 1962 to 1973, chemical synthesis was introduced as a method for producing monosodium glutamate by using acrylonitrile and dl-glutamic acid. Nowadays, MSG is produced via fermentation (due to the reduction of production costs and environmental load) by using natural products such as sugarcane and molasses. In addition, it is found in soy sauce and vegetable proteins (Jong, 2003).
Importance of glutamate at a basic function level
Glutamate occurs naturally in foods containing proteins such as tomatoes, meat, poultry, and milk. It is either bound or free. A basic function level, glutamate (monosodium glutamate) is used as a taste enhancer or for flavoring. Naturally, free glutamate in tomatoes and mushrooms is used for flavoring (International Glutamate Information Service, 2011).
Biochemical synthesis and metabolic pathways
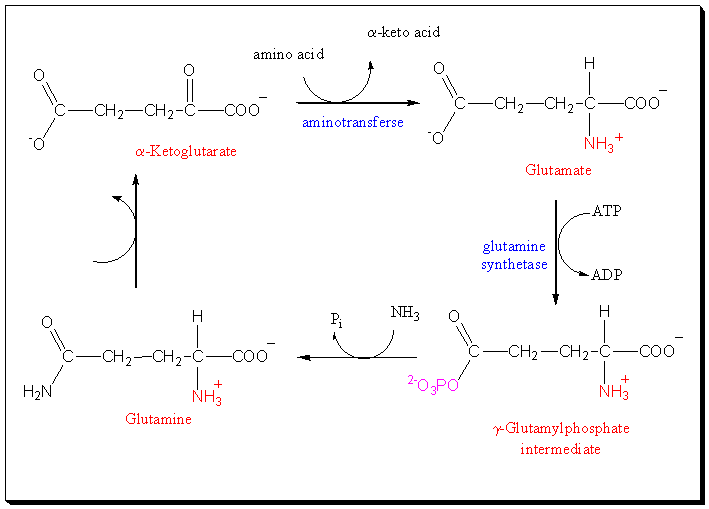
Glutamate falls under the class of non-essential amino acids synthesized from intermediates of metabolic pathways. The biochemical synthesis of amino acids is done by using α-ketoacid and transaminating from glutamate with the aminotransferase acting as a catalyst or enzyme. For glutamate, its synthesis is done by aminating its α-ketoglutarate.
Amino acids biosynthesis process takes the form:
- α-ketoacid + glutamate ⇄ amino acid + α-ketoglutarate
- while glutamate biosynthesis process takes the form:
- α-ketoglutarate + NH4+ ⇄ glutamate.
For example, glutamine is derived from glutamate and NH4+.
Aminotransferases usually make sure that any amino nitrogen is shifted to and from glutamate to enhance the dilapidation and creation of amino acids (Kelly & Stanley, 2001). Glutamate dehydrogenase releases ammonia and α-ketoglutarate through glutamate’s oxidation in the tricarboxylic acid cycle. Additionally, ammonia detoxification takes place when it is transformed into N-acetyl glutamate (NAG). Therefore, glutamate acts as a major ketoacid in many biosynthesis ways besides storing nitrogen and releasing it as urea. Feedback inhibition regulates most pathways of the biosynthesis process.
Mode of action at the cellular level
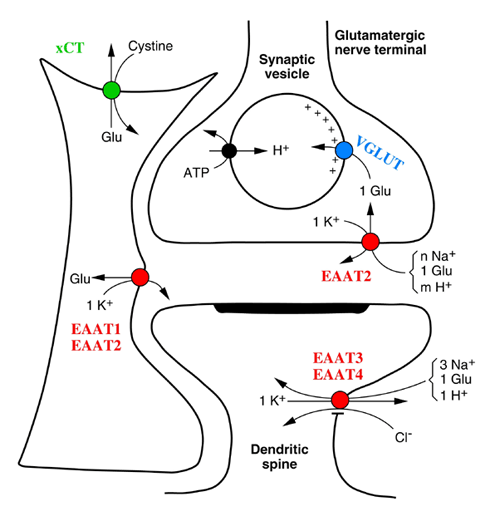
Glutamate and its receptors (on neurons and glial cells) aid in excitatory signal transmission. The major function of the receptors is to sense the cells’ release of glutamate besides converting the signal and passing it to the postsynaptic cells. Therefore, the receptors act as recognition elements and signal transducers (Gonzales & Jaworski, 1997). Glutamate found inside a cell triggers its receptors by binding to them (Danbolt, 2001). The brain networks are normally submerged in an extracellular fluid though the level of glutamate concentration in it is low. Glutamate is mostly concentrated at the nerves and synaptic vesicles.
Glutamate is removed from the fluid through an uptake arbitrated by transporter proteins which bind the molecules then move them into the glial cells and the terminals of the nerves. This is done to make sure a low concentration of glutamate in the extracellular fluid is maintained for the right functioning of the brain. The glial cells do a conversion of glutamate to glutamine while the nerve terminals convert glutamine back to glutamate and this enables glutamate to be taken back to the neurons in a non-toxic state.
Uses of glutamate molecules
Glutamate molecules have various functions and use in the human body. The molecules are used for metabolism, neurotransmission, aiding digestion, and as a flavor enhancer.
Metabolism
In cellular metabolism, glutamate is used as a major ketoacid in amino acid synthesis. The general formula for the synthesis method is:
- Amino acid + α-ketoglutarate ⇌ α-ketoacid + glutamate
Alanine and aspartate produce pyruvate and oxaloacetate respectively, which are used as substrates in glycolysis and the citric acid cycle. Moreover, glutamate is used in Nitrogen disposal. For example, ammonia is excreted from the body as urea due to its synthesis in the liver with glutamate acting as an intermediate. Cysteine and glycine merge with glutamate forming glutathione which aids in cell protection against oxidation (Andrea and Stanley, 2001).
Digestion
Glutamate, consumed from foods such as tomatoes, fish, eggs, meat, and poultry is a source of energy in the intestines hence suitable for the proper operation of the digestive system (The International Glutamate Information Service, 2011).
Neurotransmission
Glutamate molecules help in excitatory neurotransmission in the brain. Neurons use glutamate for signal transmission as well as metabolic processes (Gonzales & Jaworski, 1997). AMPA, kainate, and NMDA are glutamate receptors that bind glutamate in the postsynaptic cell while nerve impulses prompt its release this makes it important for memory, cognition, and learning (Czapinski, Blaszezyk & Czuezwar, 2005).
Flavour enhancer
Its unique test of umami makes it a good enhancer of many natural foods. Furthermore, its harmonization with salty and sour tastes makes it suitable for dressings.
GABA precursor
According to (Czapinski, Blaszezyk & Czuezwar, 2005), “In GABA-ergic neurons, glutamate acts as a precursor for GABA with the glutamate decarboxylase (GAD) acting as a catalyst in the reaction”. This prevents seizures and gabapentin which is a GABA precursor acts as an antiepileptics drug.
Diseases associated with glutamate
Stiff man syndrome and diabetes mellitus
GAD is found in the pancreas. When antibodies opposing GAD fight it, the pancreas gets destructed. The result is the disease termed diabetes mellitus. The stiff-man syndrome is also brought about by antibodies resisting GAD. This leads to stiffness in the muscles and seizures.
Cerebral ischemia, head, and spinal injury, and seizures
Glutamate is essential for brain development, cellular survival, and the creation of nerve synapses. Its excessive discharge causes diseases such as cerebral ischemia, head, and spinal injuries, and seizures. Therefore, the right quantities of glutamate should be present in the brain otherwise it becomes toxic (Danbolt, 2001).
Trauma, stress, and anxiety disorders
Glutamate is a major cause of stress and anxiety in people. Some drugs when taken harbor the transmission of glutamate thus causing stress and trauma. To make sure the disorders are eliminated, drugs taken must amend glutamatergic function (Cortese & Phan, 2005).
Parkinson’s disease
The deficiency of glutamate dehydrogenase (GDH) in a body causes Parkinson’s disease, basal ganglia disorder, and cerebella dysfunction.
References
Cortese, B.M., & Phan, K.L. (2005). The role of glutamate in anxiety and related disorders.
Czapinski, P., Blaszezyk, B., & Czuezwar, C.J. (2005). Mechanisms of action of antiepileptic drugs. Current Topics in Medicinal Chemistry, 5, 3-14.
Danbolt, N.C. (2001). Glutamate as a Neurotransmitter – An overview. In the Neurotransporter Group, Dynamics of extracellular transmitter amino acids.
Gonzales, R.A., & Jaworski, J.N. (1997). Alcohol and glutamate. Neurotransmitter review, 21, 2.
International Glutamate Information Service, (2011). History…discovery and development. Web.
Jong, S. (2003). Review on monosodium glutamates. Web.
Kelly, A. & Stanley, C.A. (2001). Disorders of glutamate metabolism. Mental Retardation and Developmental Disabilities Research Reviews, 7, 287-295.
Sano, C. (2009). History of glutamate production. Web.