Introduction
Water is one of the rare exclusions to the direct dependence between temperature and volume. The volume of water decreases upon cooling, which lasts until the temperature decrease up to about 4°C (Stoker 199). In this case, the water achieves a maximum density and, accordingly, a minimum volume. In the course of further water cooling below the mentioned temperature, it starts to expand. The identified extension endures until a triple point (O°C) when liquid water starts to change its state to solid and enlarges as well. For example, the density of ice (0.9170 g/mL) is less than that of liquid water (0.9999 g/mL) (Stoker 199). The volume increase produces a force that may negatively affect pipes and even destroy some items located in the water.
Main body
The review of the structure of water molecules shows that it has two atoms of hydrogen and one atom of oxygen (Figure 1). Since in a water molecule, two hydrogen atoms give one electron to an oxygen atom, the former receives a positive charge, and the latter – a negative one. Due to this, each oxygen atom is attracted to the hydrogen atoms of other molecules and vice versa. There is a strong tendency between atoms of hydrogen to shape a network of bonds. The expansion of water during freezing occurs due to the fact that its molecules occupy a smaller volume in an irregular arrangement compared to a completely regular structure (Stoker 199). Due to the expansion of water during freezing with increasing pressure, the freezing temperature decreases. The regular distance between water molecules proceeds to decrease along with the temperature since the kinetic energy falls.
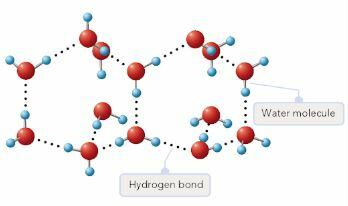
It should be stressed that hydrogen bonding may appear only in case water molecules show a certain position with regard to each other. According to Stoker, non-bonded oxygen atoms dictate the positioning and angle of water molecules (199). Consequently, water molecules tend to be far apart once they are bonded compared to the opposite state. The physical rather than thermal expansion occurs as water molecules create a crystalline structure. When oxygen and hydrogen atoms are involved in bonding, they form pairs, while one area of the water molecule receives a more positive charge compared to the other one. Thus, the molecules act in the role of triangular magnets, one side of which is electrostatically positive, and the other is negative.
Conclusion
With the lowering temperature, these bonds are likely to become stronger, thus creating a crystalline structure. The connections between freezing molecules increase, and they arrange an open structure. Since the density of solid water is lower, one may observe that ice tends to float in the liquid water (Stoker 199). When winter approaches, the process of water stratification in lakes and rivers may be described.
Namely, the so-called heaviest water is laid in the bottom, the layer with lower temperature comes next, and the top of the water is covered by ice. Such a phenomenon allows aquatic flora and fauna to exist in spite of temperature changes. Also, the natural alterations of water may be observed when potholes form in the streets as a result of the water freezing. In cold periods, car drivers use antifreeze to ensure that water in their vehicles would not freeze.
Work Cited
Stoker, H. Stephen. General, Organic, and Biological Chemistry. 7th ed., Cengage Learning, 2016.