Every organism on the planet contains water, and the presence and availability of water are necessary conditions for the development and maintenance of life (“All living things,” n.d.). Therefore, understanding processes occurring in water and understanding its characteristics in terms of interacting with other substances are crucial for understanding life itself. One of such processes is so-called osmotic pressure; i.e., the pressure needed to balance another kind of pressure, which is exerted by pure water on a water solution if they are separated by a membrane impermeable to the solute. Osmotic pressure is an important phenomenon in physics, chemistry, and biology; also, it is relevant to medicine and used in treatment planning. To explore it, it is necessary to define the term, demonstrate examples, and discuss possible applications.
Definition
First of all, osmotic pressure can be defined as “the pressure that must be applied to the solution to stop the inward flow of solvent” (Atkins & de Paula, 2013, p. 149). This inward flow is caused by the process of osmosis. The latter is the process of a liquid’s flow through a selectively permeable membrane (“Osmosis,” 2016). Such membranes allow some substances to pass through them while stopping (as if filtering) other substances. Osmosis is a crucial phenomenon because it makes such processes possible as the absorption of water by plants’ roots and the supply of oxygen and other substances to cells through the blood flow in the human organism. Osmosis causes pressure exerted by the solvent (pure water) on the solution, and the pure water flows into the solution under this pressure.
However, if certain pressure is applied to the solution, the flow of the solvent will stop; this compensation pressure is the osmotic pressure exactly. A different, more technical definition of osmotic pressure was proposed by Pfeiffer and Heremans (2012) who noted that the “use of thermodynamic quantities deviate[s] occasional[ly] from their definitions given in standard textbooks” (p. 541). These authors defined osmotic pressure as “the smallest hydrostatic pressure that is required to stop or prevent osmosis (i.e. a kind of compensation pressure)” (Pfeiffer and Heremans, 2012, p. 542). In a given case of a solution and a solvent separated by a selectively permeable membrane, the osmotic pressure (and the point at which osmosis will stop) will depend on the solute concentration in the solution.
Examples
To explain the concept of osmotic pressure, it is possible to provide an example that can be pictured. For instance, there is a U-shaped tube filled with water; at the basis of it, there is a selectively permeable membrane that allows water to flow through it but is impervious to the molecules of sugar. If sugar is added to one part of the tube, it will mean that the content of the tube is no longer homogeneous: the tube will have a sugar solution in one part of it and pure water (solvent) in the other part. As it is shown in Figure 1, in this situation, the water from the solvent part will start to flow into the solution part through the membrane: this is the illustration of osmosis. As a result, the water column in the solution part of the tube will rise, while the water column in the solvent part will drop.
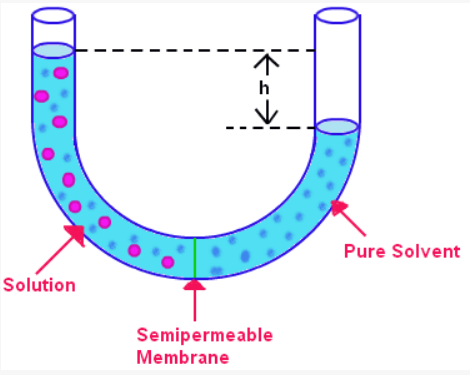
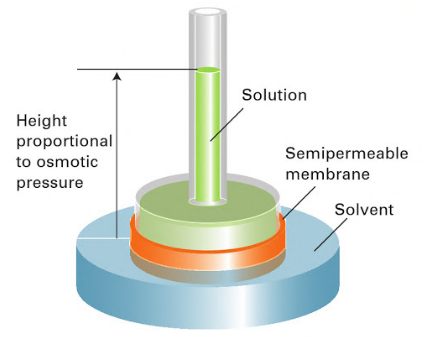
The provided example allows visualizing the pressure exerted by the solvent on the solution. In Figure 1, the osmotic pressure is the pressure that needs to be applied to the left water column (in the solution part of the tube) to stop further flow of the solvent into the solution. In a different visual example shown in Figure 2, a selectively permeable membrane is placed inside a vertical vessel. When a solute is added, the level of the solution will rise in the inner tube, as the solvent will start to flow into it. The increase in this level will be proportional to the osmotic pressure.
Application
Concerning practical applications, the notions of osmosis and osmotic pressure are particularly relevant to the way cells function. Almost all living organisms consist of cells, and processes by which cells sustain themselves, interact, and divide need to be properly understood to ensure effective medical treatment. First of all, it should be noted that cell walls are selectively permeable membranes: they allow certain substances in (such as oxygen) but prevent other important substances from leaving the cell (Kim, Park, Choi, Manoharan, & Weitz, 2014). These mechanisms allow for homeostasis; i.e., the balance among physiological processes that allow a living organism to function properly. Since cells contain water with different solutes in it and are enclosed by selectively permeable membranes, it can be concluded that they are subject to the solvent pressure and its counterforce—the osmotic pressure.
In fact, this understanding allows resolving practical issues, such as the issue of the effective use of medicines delivered through injections. If the concentration of certain solutes in the injected water solution is higher than it is inside the cells, the process of osmosis will result in an outflow of water from the cell, and such cells will shrink. In the opposite scenario, if the concentration of certain solutes in the injected water solution is lower than it is inside the cells, water will start flowing into the cells, which will make them inflate and possibly burst (Kim et al., 2014). Therefore, in order to deliver a medicine (e.g., to a person’s blood), it is necessary to prepare a solution, the content of which is based on the known content of the solution inside certain human cells. Otherwise, the process of osmosis can cause damage to the cells, which may lead to other health problems and complications.
Conclusion
Osmotic pressure is the pressure that needs to be applied to a solution separated from a solvent by a membrane impervious to the solute in order to stop the process of osmosis. Initially, osmosis causes the solvent to flow into the solution because it is a natural rule that the substance of a lower solute concentration will flow into the substance of a higher solute concentration. In a U-shaped tube with a selectively permeable membrane separating pure water from a sugar solution, the solution column will rise, and the pure water column will drop. Osmotic pressure in this example needs to be applied to the solution column to stop osmosis. A proper understanding of these processes allows resolving practical issues, such as the issue of preparing solutions that will carry medicines to a human body through an injection.
References
All living things need water. (n.d.). Web.
Atkins, P., & de Paula, J. (2013). Elements of physical chemistry (6th ed.). Oxford, England: Oxford University Press.
Kim, S. H., Park, J. G., Choi, T. M., Manoharan, V. N., & Weitz, D. A. (2014). Osmotic-pressure-controlled concentration of colloidal particles in thin-shelled capsules. Nature Communications, 5(3068), 1-22.
Osmosis. (2016). Web.
Osmotic pressure=hydrostatic pressure in Ushaped tube. (2015). Web.
Pfeiffer, H., & Heremans, K. (2012). On the use of the term osmotic pressure. In R. Winter & J. Jonas (Eds.), High pressure molecular science (pp. 541-546). Norwell, MA: Kluwer Academic Publishers.