Introduction
With the help of chemistry, we have been able to define at the molecular level what is happening in and around us chemically. Take for example a freshly-cut apple turns brown, an iron nail exposed to air becomes rusty and a copper coin all of a sudden turns green. The process in which living systems produce and utilize energy, photography, and the operation of a car battery, are all common examples of a group of chemical reactions called the oxidation reaction.
Definition of oxidation
By definition, oxidation is the interaction between the substances from metal to living tissues with oxygen molecules. Oxidation reactions can be either constructive or destructive. For instance, the oxidation processes involved in photosynthesis are essential reactions for the survival of entire living organisms. Sometimes oxidation can be destructive, for instance when we see our car rusting or the fresh fruits spoiling we feel bad. These are some of the inevitable reactions occurring in nature through the process of oxidation.
While oxygen we breathe is an essential part of our life it is also a cause for unwanted oxidation reactions. The amount of oxygen present in the atmosphere and the nature of the material it comes in contact with determine the process of oxidation. Though true oxidation happens on a molecular level, we can see without naked eyes only the large-scale effects of the oxygen. Nature offers protection against oxidation for instance the skin in the case of fresh fruit provides a barrier against oxidation. Only once the fruits such as an apple when it is cut the individual cells come in direct contact with air and the oxygen molecules start burning it. This is when we can see the effects of oxidation within minutes as brownish spots or blemishes (Pollick n. pag).
Reduction reactions
Reduction reactions also play important role in our daily life. While oxidation reactions are characterized by the addition of oxygen, reduction reactions are characterized by the removal of oxygen.
Take for example the reaction between metallic magnesium and oxygen from magnesium oxide. This reaction involves the oxidation of magnesium.
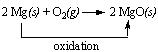
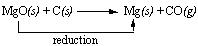
On the other hand, when the reverse process occurs i.e. when magnesium oxide is converted to metallic magnesium, a reduction reaction occurs where the oxygen is removed. For instance, the reaction between magnesium oxide and carbon at 2000C gives rise to magnesium metal and carbon monoxide. This is an example of the reduction of magnesium oxide to magnesium metal. The following is the reduction reaction that occurs.
The role of electrons
Based on the above-mentioned examples, oxidation in simple terms can be defined as, the “addition” of oxygen; and reduction, as the “removal” of oxygen (Asato n. pag).
With the discovery of electrons, the oxidation and reduction reactions were defined in terms of the transfer of electrons. Scientific research could prove that oxidation-reduction reactions involved the transfer of electrons from one atom to another (Hill and Kolb 2006). Hence under this new concept, the above-mentioned oxidation reaction of magnesium and oxygen can be represented as follows.
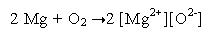
If we further break this reaction it can be noted that each magnesium atom loses two electrons to form an Mg2+ ion. The following reaction occurs
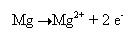
And, each oxygen molecule gains four electrons to form a pair of O2- ions.
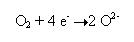
However, the above-mentioned two reactions occur simultaneously because electrons are neither created nor destroyed in a chemical reaction. If we carefully observe the two reactions it can be noted that the first one is an oxidation reaction and the second one is a reduction reaction. Hence oxidation and reduction are linked. It will be more clear in the following reaction.
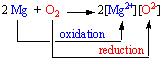
Since both reduction and oxidation are going on simultaneously, this is known as a redox reaction (Bodner research web n pag).
Examples of oxidation and reduction
Let us take another example of extraction of iron from its ore. The following reaction occurs:
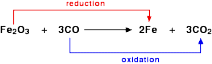
There is another way in which oxidation and reduction reactions can be defined. Oxidation takes place when the oxidation number of an atom becomes larger, whereas reduction takes place when the oxidation number of an atom becomes smaller. For example, consider the following reaction.
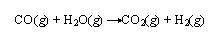
This reaction can be further represented diagrammatically as follows:
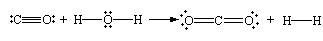
From this figure, it can be noted that the total number of electrons in the valence shell of each atom remains constant in this reaction. The oxidation state of each of these atoms changes in the reaction. The oxidation state of carbon increases from +2 to +4, which is the characteristic of oxidation reaction, and the oxidation state of the hydrogen decreases from +1 to 0 which is the character of a reduction reaction (Bodner research web n page). The above-mentioned reaction can also be illustrated as follows:
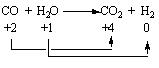
Let us consider some more simple examples of oxidation and reduction reactions. In the burning of hydrogen, the hydrogen is oxidized and the oxygen is reduced.
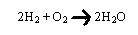
The reaction between nitrogen and oxygen which occurs at high temperatures is another oxidation-reduction reaction. In this case, the formation of nitric oxide is through the oxidation of the nitrogen and reduction of the oxygen.

There are also examples where, the oxidation is most prominent. For example, in the burning of methane, both carbon and hydrogen are oxidized or gain oxygen.
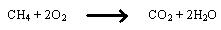
Reduction reaction can also be defined as gaining of hydrogen.
Lead dioxide at high temperatures appears to be just reduction reaction. The following reaction occurs
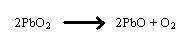
In this case the reduction of the lead dioxide is clear. However, oxidation in this reaction is easier to see when we describe oxidation as the losing of electrons (Hill and Kolb 2006).
Oxidizing Agents and Reducing Agents
Some substances can oxidize other substances or in other words help the oxidation reaction to occur. These substances are known as oxidizing agents, oxidants, or oxidizers. In terms of the electron, the oxidant or the oxidizing agent removes electrons from another substance whereby it reduces itself by accepting electrons. And hence these oxidizing agents are also called electron acceptors. Oxidizing agents are characterized as substances with elements in high oxidation numbers. Some of the common oxidizing agents are H2O2, MnO4-, CrO3, Cr2O72-, OsO4, or substances that are highly electronegative that can gain one or two extra electrons by oxidizing a substance such as O, F, Cl, Br.
In contrast to oxidizing agents, those substances that can reduce other substances are known as reducing agents, reductants, or reducers. In terms of the electron, the reductant or the reducing agents transfers electrons to another substance where it oxidizes itself by donating electrons. And hence these reducing agents are also called electron donors. Reducing agents are very diverse. Electropositive elemental metals such as Li, Na, Mg, Fe, Zn, and Al are examples of reducing agents which donate or give away electrons readily. Other examples of reducing agents include NaBH4 and LiAlH4, these are reducing reagents widely used in organic chemistry. Reductions using hydrogen gas (H2) with palladium, platinum, or nickel catalyst are also examples.
Applications
Oxidation-reduction reactions are so common in our lives that we ignore most of these reactions. Besides these are some of the important reactions that have many important applications in our lives. The following are just a few examples of oxidation-reduction reactions that occur in and around us.
Bleaching agents are compounds that are used to remove the color from substances such as textiles, teeth, floor, etc. Traditionally bleaching of textiles was done by simple exposure to the sun and air but today most commercial bleaches are oxidizing agents, such as sodium hypochlorite (NaOCl) or hydrogen peroxide (H2O2). These are quite effective in “decolorizing” substances via oxidation.
Photosynthesis: It is an example of naturally occurring biological oxidation-reduction reactions. The reactions involved in the preparations of food are a very complex process carried out by green plants, blue-green algae, and certain bacteria. These organisms utilize the energy contained in sunlight, and through a series of oxidation-reduction reactions, produce oxygen and glucose. In a simple reaction the process of photosynthesis can be represented as follows:
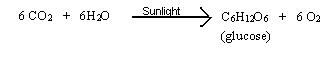
Photosynthesis involves two basic sets of reactions called the light reaction and the dark reaction. The light reaction is the splitting of water which is an oxidative process occurring in the presents of sunlight. This reaction may be written as:
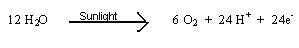
Along with the oxidation of water, there is another reduction reaction that occurs that results in the formation of a compound called nicotinamide adenine dinucleotide phosphate (NADPH). The following reaction occurs:
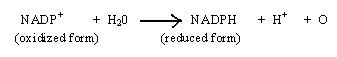
Along with the above reaction there is another reaction that occurs which results in the formation of a high energy compound, called adenosine triphosphate, (ATP). All these reactions combine to form the light reaction.
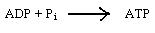
The dark reaction utilizes the energy contained in both NADPH and ATP to reduce carbon dioxide to a type of sugar (C6H12O6) called the glucose.
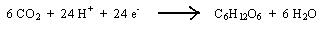
There are several other examples of oxidation and reduction processes such as the metabolism, combustion process, nitrogen fixation etc. (Asato n. pag).
References
Asato, R. Oxidation/Reduction Internet chemistry, 2007. Web.
Bodner research. Oxidation and Reduction. 2007. Web.
Hill, J.W. and Kolb, D.K Oxidation and Reduction: Burn and Unburn, In: Chemistry For Changing Times (11th Edition) Prentice Hall (2006).
Pollick, M. What is Oxidation? 2007. Web.
Redox. 2007. Wikimedia Foundation, Inc. Web.