Introduction
Petroleum products provide for the vast majority of man’s energy needs. These products are used to power industries, transportation, and to provide heat. Petroleum products are obtained from fossil fuels which have to be refined to be converted into products that can be put into various uses. One of the processes carried out during refining is hydrotreating. Speight and Ozum assert that the use of hydrogen in thermal processes is the single most significant advance in petroleum refining technology during the 20th century (472). This paper will provide a brief but informative description of the hydrotreatment process. The paper will begin by defining this process and highlighting the objectives of the hydrotreatment process.
Definition of Hydrotreatment
Hydrotreatment refers to a series of hydrogenation reactions subjected to crude petroleum and other refinery streams to remove impurities or saturate a variety of unsaturated hydrocarbons contained in the crude petroleum. Maples asserts that the hydrotreatment process is essential for all refining plants that convert various fossil fuels into transportation fuels (472). Hydrotreating is becoming more necessary as the supply of light crude oils becomes scarce. Heavy sour crudes are becoming more prevalent and these products contain many undesirable components including nitrogen, iron, and iron compounds. Through hydrotreating, the petroleum residents, which are the residual portion of the initial atmospheric crude oil distillation, can be converted into viable diesel and other lighter fuels (Speight and Ozum 470).
Objectives of the process
Hydrotreating is carried out to achieve several important objectives. The first major objective of hydrotreatment is to remove impurities from petroleum products. These impurities, which include sulfur, nitrogen, and oxygen, reduce the efficiency of the final products from the refining process. Lawrie states that hydrotreatment results in the conversion of organic sulfur, nitrogen, and oxygen compounds to hydrocarbons and hydrogen sulfide, ammonia, or water (223). Through hydrotreatment, the impurities are removed for the control of a final product specification.
The process also aims to change the composition of some renewable fuels to make them similar to fossil fuels. By doing this, hydrotreating makes it possible for biofuels to be used in place of fossil fuels without having to modify the vehicle’s engine (Lawrie 225). This is achieved since the process changes the composition of the renewable fuels into a form that is similar to normal fossil fuels used by vehicles.
Another objective is to remove metals from petroleum products. This objective is fulfilled in a separate guard catalytic reactor where the metallic compounds contained in the petroleum are hydrogenated and decomposed to create metal deposition. Maples reveals that with the increasing mandates in many countries for the burning of cleaner fuels, hydrotreating has become essential in the removal of heavy metals to produce a cleaner-burning fuel (243).
Hydrotreatment is done to convert inferior or low-grade materials into valuable products. Saturate olefins cannot be used as fuel in their unstable compounds. Through hydrotreating, unsaturated compounds are transformed into the corresponding saturated hydrocarbons. This transformation enables the otherwise unusable saturates to be used as fuel (Maples 250). Through hydrotreatment, the productive life of oil, which is our society’s main energy source, is optimized.
Hydrotreatment ensures that the various products of crude oil such as gasoline, kerosene, and diesel oil meet the various standard specifications. The increase in fuel oil demand and the gradual decrease in the availability of lighter types of oil has meant that heavier feedstocks have to be used. As the fossil fuel reserves are used up, the quality of the world’s crude oil will decrease even further with time. Hydrotreating is the only way that clean transportation fuels can be produced from these heavy feedstocks (Maples 250). For this reason, this process is regarded as one of the most important catalytic processes in oil refineries.
Hydrotreatment Process
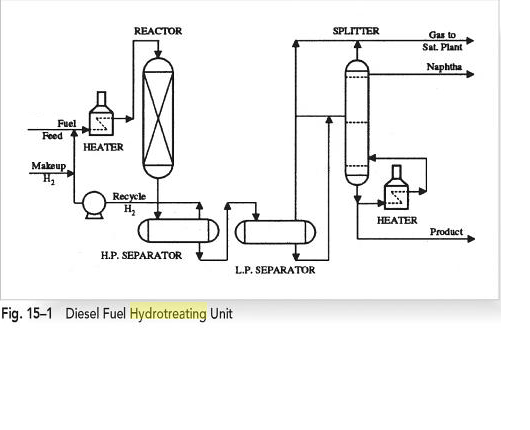
A typical hydrotreater operates in the following way. The reactor has a feed that contains the fuel that is to undergo the hydrotreatment process. The fuel feed stream is combined with recycled hydrogen and makeup hydrogen. This combination of hydrogen and fuel is heated in a fired heater as it heads to a fixed bed reactor. The effluent obtained from this reaction is separated in a high-pressure separator into a liquid phase and a recycled hydrogen stream (Lawrie 225). The liquid, which is at high pressure, is flashed into a low-pressure separator producing gas and a liquid feed. The gas is directed to a gas plant to recover hydrocarbons while the liquid is fractionated into a hydrotreated product and lower boiling material produced in the reactor.
Feeds Used
The feed stream is generally made of any petroleum stream that is in need of further treatment. Straight-run naphthas, which are intermediate hydrocarbon streams, are the most prevalent feeds for hydrotreaters. Naphthas are obtained from refining crude oil, coal tars, a distillation of wood, or coal gasification. Due to this wide variety of sources, as well as the unique chemical makeup of crude oil from various regions, the composition of naphthas differs significantly (Lawrie 225).
Middle distillates such as diesel, kerosene, and jet fuel are also used as feed. Hydrotreatment in this case aims to remove sulfur mostly for environmental reasons (Maples 250). In addition to this, the process increases the cetane number by hydrogenating aromatic components in the feed. Cracked gasoline can also be used as a feed. When this fuel is used, the aim of the process is to hydrogenate undesirable sulfur and nitrogen compounds contained in the cracked gasoline.
Fuels derived from renewable organic material are also used as feed for hydrotreaters. Hydrotreatment is necessary to convert the feedstock obtained from renewable organic matter into fuels that can be used in vehicles.
Operating conditions
Hydrotreating operations are carried out at different conditions depending on the type of feed used. However, hydrotreatment generally occurs at very high temperatures and pressure to promote the kinetics of a number of the reactions that occur during this process (Maples 255). For naphtha feeds, the reactor temperature is between 280° and 425°C while the pressure is at 200 to 800 (psig). When using gas oil feeds, reactor temperatures are between 340°-425°C and the pressure is between 800 and 1600 psig (Maples 257). For resid, the operating temperatures are 340°-450°C while the pressure is between 2000 and 3000 psig.
Catalyst
Catalysts play a crucial role in the hydrotreating process. Some of the traditional catalysts used were nickel/molybdate. These catalysts are active for the hydrogenation of nitrogen compounds to ammonia and a hydrocarbon. They also produce some saturation of olefins and aromatics. To increase the efficiency of catalysts, modern refineries also make use of cobalt/molybdate. Use of both cobalt/molybdate and nickel/molybdate catalysts results in greater purification of various crude oil fractions (Lawrie 223). A layer of relatively inert spheres that are packed into the reactor supports the catalyst. When using heavy feeds, the catalysts’ pores at the top of the bed become blocked with metals and the high molecular-weight hydrocarbons present in the feed. This leads to a reduction in the catalyst activity and the catalyst must be regenerated in order to resume optimal efficiency.
Main Reactions
The reactions during the hydrotreatment process are mostly exothermic. This means that the reactor temperature rises as new feed goes through the hydrotreater. A major reaction is desulphurization, which is the removal of sulfur from the feed by reacting a combination of the feed and hydrogen in the presence of a catalyst. This process requires high temperatures and hydrogen pressures. Another reaction is between Hydrogen and nitrogen compounds contained in the feed. The reaction takes place between Hydrogen and the Nitrogen compounds to form NH3 which can be removed together with other gaseous products. Reactions also occur between hydrogen and impurities such as coke and metals in the presence of a catalyst. This reaction results in the deposition of coke and mental compounds on the catalyst.
Hydrotreating can be used to convert hydrocarbons by changing their molecular structure. This is achieved by reacting hydrogen with hydrocarbons to reduce the molecular weight of the hydrocarbons. When this occurs, heavy hydrocarbons can be converted into light hydrocarbons that can be used as engine fuels.
Main Products
There is a wide array of products that can be obtained from the hydrotreatment process. In gas plants, LPG and Fuel gas are produced. These plants also produce gasoline (Speight and Ozum 466). Crude oil produces various grades of kerosene including mid-distillate kerosene and naphtha kerosene. Paraffins are also obtained from hydrotreating biofuels. At a higher temperature, crude oil products including Gasoline and diesel oil are produced as the finished products from the hydrotreatment process. At the highest temperature, lubricating oil and grease are produced
Technologies
Hydrotreating technology has not undergone many changes since its invention more than 6 decades ago. The process is still based on passing the feed and hydrogen through a catalyst at elevated temperatures and pressure. However, different technologies have been invented to increase efficiency. For the processing of petroleum resides the three-phase ebullated beds’ reactor is preferred (Speight and Ozum 470). This hydrotreater comprises a liquid phase, an inlet gas phase, an outlet gas phase, and solid-phase catalysts. A significant technological advancement in hydrotreaters has been the introduction of bimodal catalysts. Unlike typical catalysts that have uniformly sized pores, bimodal catalysts contain both large and small pores (Speight and Ozum 471). These catalysts overcome the problem of blocking caused by heavy feeds. As a result, the operating cycle of the catalyst is extended since it takes longer before the heavy feed block the catalyst with metal.
Conclusion
This paper set out to describe hydrotreatment, highlight the objectives of the process, and elaborate on how it is carried out. It began by underscoring the importance of the hydrotreating process in petroleum refining. The paper has noted that hydrotreating is done for environmental and economic reasons. Through this process, oil refiners can utilize the available fossil fuels optimally. The paper has articulated the role that hydrotreating plays in the quality improvement of petroleum products. It can be expected that this process will continue to play a major role in petroleum products processing.
Works Cited
Lawrie, Lloyd. Handbook of Industrial Catalysts Fundamental and Applied Catalysis. NY: Springer, 2011. Print.
Maples, Robert. Petroleum Refinery Process Economics. London: PennWell Books, 2000. Print.
Speight, James and Ozum Baki. Petroleum Refining Processes. Boston: CRC Press, 2001. Print.