Introduction
Pfiesteria piscicida is a single-cell, a microscopic organism found in the environment for millions of years (Goodis 369). Pfiesteria piscicida is a comparatively small, lightly armored, heterotrophic dinoflagellates found commonly in estuarine waters (Steidinger et al. pp. 661-665, Parrow and Burkholder pp. 678-696). This paper focuses to bring out classification, ancestry, and evolution, adoptions, life cycle, and behaviors of Pfiesteria piscicida. According to Burkholder Pfiesteria piscicida has always been naturally present in the estuaries and tidal rivers of North Carolina. It is only recently about a decade back that these have become a serious cause of concern due to their morph into a toxic fish killer. Since its discovery, Pfiesteria piscicida it is reported that it has killed more than one billion fish (Barker).
Classification
Dinoflagellates are a group of microscopic one-celled microorganisms. These are in general classified both botanically and zoologically, but they are typically put in the botanical division Pyrrhophyta. Pfiesteria and Pfiesteria-like species are thinly armored forms with motile dinospore stages characterized by their distinct plate formulae. Pfiesteria piscicida is the best-known member of the genus (Steidinger et al. pp. 661-665). Most are free-swimming and are plant-like, that is, they can obtain energy by photosynthesis. Though in general dinoflagellates are considered nontoxic organisms, Pfiesteria piscicida, is an exception to that rule. It is toxic and can be both plant-like by performing photosynthesis and animal-like by consuming other organisms (mtholyoke.edu).
Evolution
Several researchers have worked on the evolutionary process of Pfiesteria piscicida. Dinoflagellates are believed to have close links with ciliates and apicomplexans at the ultrastructure level. This is mainly because of the similar organelle structures like the tubular mitochondrial cristae, type of spindle, rod-shaped trichocysts, and cortical vesicles, and also similarities at the genetic level (Litaker et al. pp. 1379–1389). Additionally, Dinoflagellates specifically have a dikaryon nucleus with continually condensed chromosomes all through their life cycle.
With the support of the fossil record, Fensome et al. proposed a possible phylogenetic scenario or tree. According to this, structurally, unarmored dinoflagellates with unfilled cortical vesicles (hundreds) preceded armored forms in the evolutionary process. Later the armored forms evolved from groups having many plates i.e. vesicles filled with polysaccharide plate material to those having only a few plates that are commonly found in the order Prorocentrales.
Several arguments still need to be satisfied. According to this evolutionary process, the placement of Pfiesteria and related genera depends on several attributes that need further clarification. Initially, Pfiesteria was placed in the order Dinamoebales, which incorporates species with several life-history stages, together with dominant amoeboid forms. But, there are concerns that the dominant life history stage is a motile dinospore or coccoid stage with closer morphological affinities to the order Peridiniales. Researchers have proposed to transfer Pfiesteria from the order Dinamoebales to the order Phytodiniales or to place Pfiesteria specifically into the order Blastodiniales. Recent phylogenetic work on the rRNA ITS and 5.8S regions agree to the placement of Pfiesteria in the Peridiniales or a group between the Peridiniales and the Blastodiniales (Steidinger et al. pp. 661-665).
Discovery
Even though it is believed to have been in the environment for millions of years, Pfiesteria piscicida was only recently discovered. In the year 1988, Dr. JoAnn Burkholder, who is an aquatic ecologist at North Carolina State University, discovered the organism and declared it a new species in a new genus and a new family in the order Dinamoebales. Later Burkholder and her associates named the new organism Pfiesteria after the late Dr. Lois Pfiester, who was a researcher of dinoflagellates. Additionally, the term “pesticide” part means “fish killer.” Pfiesteria piscicida may impersonate as a plant when they appear to photosynthesize, or it may also feed on bacteria and algae (mtholyoke.edu).
Studies based on advanced DNA analyses show that Pfiesteria piscicida lacks a certain genetic structure necessary for making the type of toxic proteins linked with typical dinoflagellates. Studies conducted by NOAA National Centers for Coastal Ocean Science, the National Institute of Standards and Technology, the Medical University of South Carolina, and the College of Charleston (S.C.) have isolated and characterized the toxin in the estuarine dinoflagellate Pfiesteria piscicida. They have also identified the process by which this organism transforms from a non-toxic to toxic state (Peter et al, pp. 1166 -1172).
Life Cycle
Several researchers have worked on the lifecycle of Pfiesteria piscicida. However, different researchers have arrived at different life stages and as a result, there exists a real confusion in this matter. While some research especially dinoflagellate experts have shown the living cycle of Pfiesteria Piscicida is extremely complex involving at least 24 different stages from cyst to several amoeba-like forms, others have found only a simple life cycle with no toxic amoebic stage. While the original research argued that the organism moves through many different stages as environmental conditions demands. It is only the recent research in 2002 that claims the cycle is much simpler than thought earlier, and that the true Pfiesteria is non-toxic. However, the microscopic and chemical identification methods used do not differentiate toxic from non-toxic strains. Other methods such as fish bioassays are available to find out if the strain is a fish-killer (Burkholder et al. pp. 745-756).
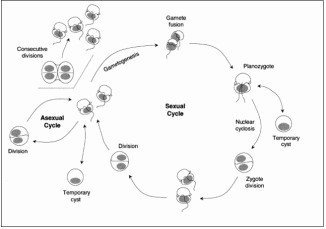
A study by Parrow and Burkholder (664–673) on the sexual life cycle events in Pfiesteria piscicida and cryptoperidiniopsoid heterotrophic dinoflagellates was carried out. They found that the observed sequence of zygote formation, development, and postzygotic divisions in both these dinoflagellates (Figure 1). The fusion of motile gamete pairs each produced a rapidly swimming uninucleate planozygote with two longitudinal flagella. This was followed by the planozygotes enlarging as they fed constantly on cryptophytes. In less than 12 hours it was found that each planozygote formed a transparent-walled non-motile cell (cyst) with a single nucleus. However, these zygotic cysts did not display dormancy under these conditions. The researchers found that there were dramatic swirling chromosome movements (nuclear cyclosis) in zygote nuclei before division. Only after the nuclear cyclosis, a single cell division occurred in P. piscicida zygotic cysts, producing two offspring that emerged as biflagellated cells. Further, the two flagellated cells swam for hours and fed on cryptophytes before encysting. A single cell division in these cysts produced two biflagellated offspring that also fed before encysting for further reproduction. This series of zygote development and postzygotic divisions was completed within 24 hours (Parrow and Burkholder, pp. 664–673).
Distribution
Pfiesteria piscicida is a common, small dinoflagellate found in estuaries around the world. Until recently researchers have identified Pfiesteria piscicida from North America, South America, Mexico, Europe, Australia, and New Zealand. Rublee et al. 2004 stated that “The widespread distribution suggests that Pfiesteria species are endemic and generally benign members of estuarine communities since impacts on fish and human health have not been reported outside the USA coastal waters” (cited in Parrow and Burkholder, pp. 664–673).
Fish kills and human health
Though several researchers are working on the impact of Pfiesteria piscicida on human health, results are still not confirmed. They have also put forward the likelihood that toxins produced by these dinoflagellates might affect human health either directly by exposure in the environment or indirectly by consumption of fish or shellfish. According to the U.S. Environmental Protection Agency “there is no evidence that shows that people can get sick from eating fish or shellfish that have been caught in coastal waters that contain toxic Pfiesteria piscicida“9. However, the agency goes on to warn that to be safe, people should avoid eating fish with lesions (The Division of Water Quality).
It is found that Pfiesteria piscicida can survive for long periods in sediments in a cyst or spore form and experiences a large population boom. This is the time when fishes in large concentration in the calm, shallow waters are affected by this organism which in its active free-swimming form produces a toxin and causes deep, ulcerative lesions in fish and kill them. However, the free-swimming form of Pfiesteria piscicida that produces the toxin exists for a very short time during the fish kill. Pfiesteria piscicida is generally considered harmless in other life stages and may spend life feeding on bacteria and algae. Scientists have pointed out that environmental factors induce Pfiesteria piscicida to change into its toxic form, as it has been shown in the laboratory that blooms of the free-swimming toxic form are mainly due to nutrient enrichment and the presence of large amounts of fresh fish excreta (Maryland Department of Environment). Therefore, it can be said that though Pfiesteria piscicida, in general, is a harmless organism except in its active free-swimming form, research is essential that will aid to prevent the fish kills and human impact
Habitat
Pfiesteria piscicida is found in both estuarine and in subtropical to cold temperate environments. Since these species have a wide salinity tolerance and are heterotrophs, it is also possible that they have a more widespread distribution. It is noted by researchers that when suitable food is no longer available, feeding, motile cells in the water turn back into resting stages. In general, prey items include pigmented microflagellates, bacteria, ciliates, or other microbiota, and even larger animals such as menhaden fish or shellfish. These dinoflagellates thrive well in nutrient-enriched waters.
Summary
Pfiesteria piscicida though present in the marine environment, it is only recently that these organisms have been identified for their morph into a toxic fish killer. As of today, it is said that Pfiesteria piscicida has killed more than one billion fish, after its discovery. Pfiesteria piscicida is classified as a microscopic estuarine dinoflagellate protozoan. In the evolutionary process, dinoflagellates are believed to have close links with ciliates and apicomplexans at the ultrastructure level. Though earlier Pfiesteria was kept in the order Dinamoebales incorporating species with some life-history stages, together with dominant amoeboid forms, it is only recently that researchers have planned to transfer Pfiesteria from the order Dinamoebales to the order Phytodiniales or to place Pfiesteria specifically into the order Blastodiniales. Pfiesteria Piscicida is extremely complex involving at least 24 different stages from cyst to several amoeba-like forms. These organisms are now known to produce toxins affecting the fish population and also might affect human health either through the consumption of affected fish or through direct exposure.
References
- Barker, R. And the Waters Turned to Blood: The Ultimate Biological Threat. Simon & Schuster, 1997, New York.
- Burkholder, J.M., Marshall H.G., Glasgow H.B., Seaborn D.W, and Dreamer-Melia N.J.. The standardized fish bioassay procedure for detecting and culturing actively toxic Pfiesteria, used by two reference laboratories for Atlantic and Gulf States. Envir. Health Perspect. (2001) 109:745-756.
- Fensome R.A., Taylor F.J.R., Norris G., Sarjeant W.A.S., Wharton D.I. and Williams G.L. A Classification of Fossil and Living Dinoflagellates. Micropaleont Species, Publ 7. (1993) New York:American Museum of Natural History.
- Goodis, J. Hear Leading Expert on Pfiesteria 1997, EPA Environmental News 369. Web.
- Litaker R.W., Tester P.A., Colorni A., Levy M.G. and Noga E.J. The phylogenetic relationship of Pfiiesteri piscicida, cryptoperidiniopsoid sp., Amyloodinium ocellatum and a Pfiiesteri-like dinofllagellat to other dinoflagellates and apicomplexans. J Phycol (1999) 35:1379–1389.
- Maryland Department of Environment Pfiesteria Fact Sheet.
- mtholyoke.edu Pfiesteria piscicida: A Toxic Dinoflagellate Plaguing North Carolina.
- Parrow, M.W., and Burkholder J.M. Estuarine heterotrophic cryptoperidiniopsoids (Dinophyceae): life cycle and culture studies. J. Phycol. 2003, 39:678-696.
- Parrow M.W., and Burkholder J.M. The Sexual Life Cycles of Pfiesteria Piscicida and Cryptoperidiniopsoids (Dinophyceae), J. Phycol. (2004) 40, 664–673.
- Peter D. R. et al. Metal Complexes and Free Radical Toxins Produced by Pfiesteria piscicida. Environ. Sci. Technol., (2007) 41 (4), 1166 -1172.
- Steidinger, K., J. et al. Classification, nomenclature, and identification of Pfiesteria and Pfiesteria- like species. Environ. Health Perspect. 2001.109 (suppl 5):661-665.
- The Division of Water Quality, Fact Sheet: Estuarine Fish Kills in North Carolina.
Definitions
- Cryptosporidium:Cryptosporidium spp. are apicomplexan parasites that inhabit the brush-borders of the gastrointestinal epithelium.
- Heterotrophic: These are organisms that cannot synthesis their own food and are dependent on complex organic substances for nutrition.
- Planozygote: When conditions become unfavourable and when nutrients become depleted or there is insufficient light, some dinoflagellate species alter their life cycle dramatically. Two vegetative cells will fuse together and this condition of a dinoflagellate is called planozygote.
- Dinokaryon: Most dinoflagellates are distinguished by a dinokaryon, which is a special eukaryotic nucleus involving, among other distinctive features, fibrillar chromosomes that remain condensed during the mitotic cycle. The dinokaryon is surrounded by a perforate nuclear envelope which does not break down during cell division. The mitotic spindle is extranuclear. The dinokaryon contains from five to ten times the levels of DNA usually found in eukaryotic cells. It lacks histones and therefore has no nucleosome.