- Produce a figure with an appropriate figure legend of your TLC plate that could be published in a scientific journal.
- Indicate all relevant information, e.g.origin, solvent front. You can use e.g. Power-Point, Photoshop or Illustrator or similar software for his task
- Identify and label the different pigments observed
- Identify the samples spotted in the different lanes.
- Write a concise figure legend with a minimal number of words that allows the reader to understand what he sees. Note: Do not repeat all details of the methods in the legend!
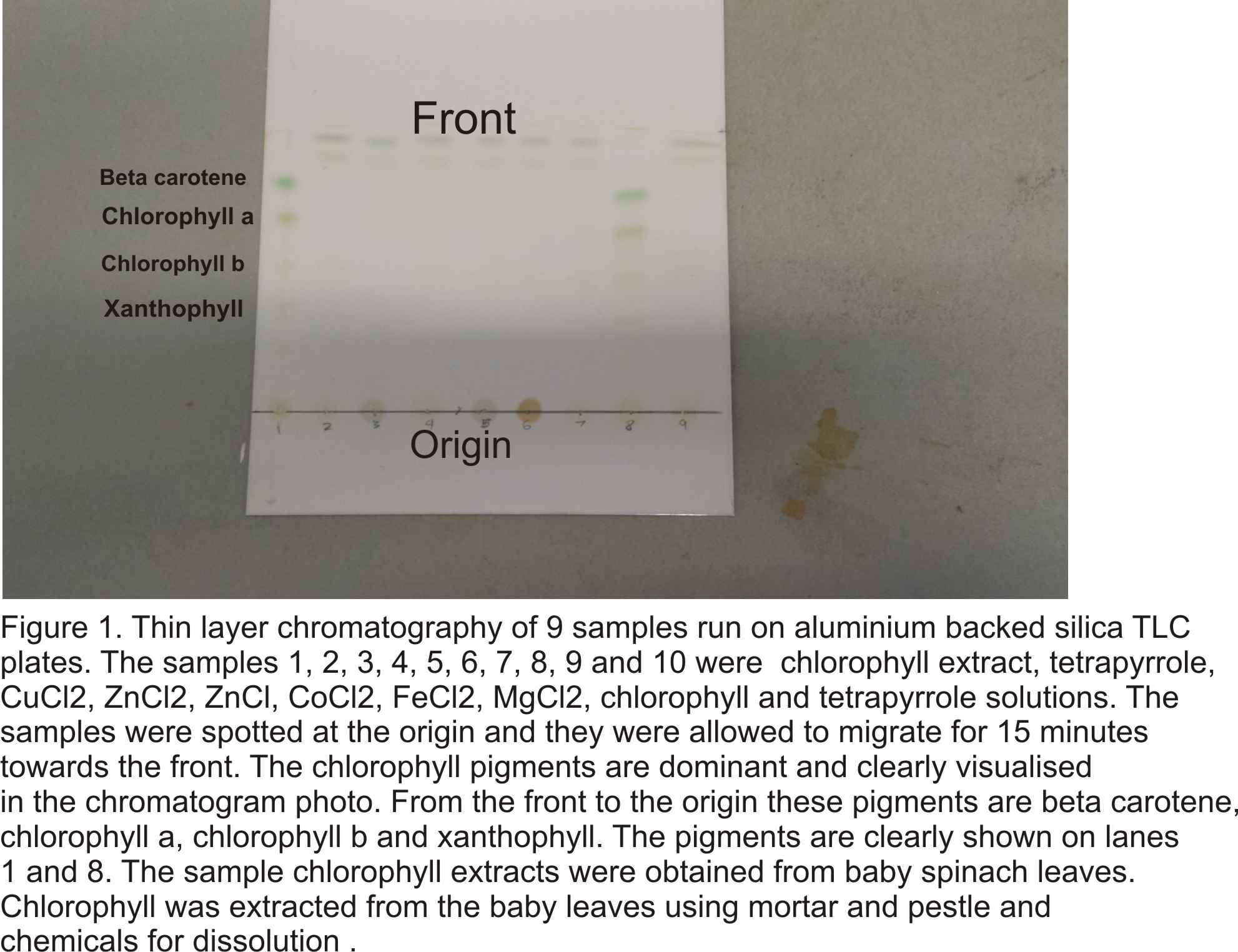
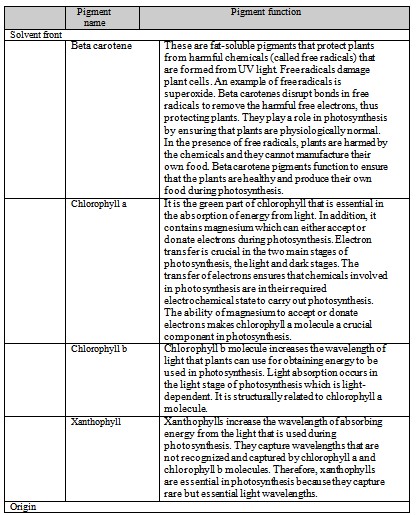
- 2. What pigments were observed in the chlorophyll extract? What is the function of each of these pigments?
- What does the removal of Mg2+from the tetrapyrrole ring using cation exchange chromatography tell you about the bonds holding the Mg2+ in place?
The removal of Mg2+ implies that the bonds holding these cations onto the tetrapyrrole ring are weak. Mg2+ ions are positively charged ions found in the tetrapyrrole ring in the chlorophyll structure. The resin utilized in the strong ion-exchange chromatography contained negative charges. The negatively charged molecules strongly attracted the positively charged Mg2+ from the tetrapyrrole ring. Therefore, it is easy for the anions in the chromatography resin to attract and remove the Mg2+ from the ring. It has been demonstrated that chemical bonding plays a crucial role in the structural integrity of compounds. Compounds with weak chemical bonds are easily destabilized by other chemicals. On the other hand, compounds with strong chemical bonds are strong and are not easily destabilized by other chemicals.
- Why are the chlorophyll pigments green?
Chlorophyll pigments absorb light at specific wavelengths. The pigments capture violet to blue (400-500 nm) and orange to red (650-700 nm). It has been demonstrated that chlorophyll pigments absorb colours at different light wavelengths. However, green light is not absorbed by the pigments. It is reflected by chlorophyll pigments. The reflection of green light by chlorophyll pigments makes them appear green. The spectrum at which chlorophyll pigments absorb light energy from the sunlight helps categorise them.
- A compound is added to the coupled photosystem of plants that binds to the reduced plastoquinone(PQH2) produced by photosystem II and prevents it from interacting with the other components. Briefly explain what this would do to the functioning of the coupled photosystem and to the plant. Assume that there is excess compound compared to the amount of plastoquinonepresent.
Photosystem II in photosynthesis is started when charges are separated between P680 and pheophytin. The process of charge separation occurs quite fast to ensure quick and constant transfer of components released in light and dark stages of photosynthesis. Plastoquinone is essential in preventing charges from recombining in photosystem II. The binding of the inhibiting compound will prevent plastoquinone from detaching from reaction centre into the inner water hating part of the membrane. When plastoquinone accepts an electron at the QA-site, it is moved to a different chemical molecule at the QB-site. The photosystem II process is maintained by cycling of electrons and protons between the QA-site and QB-site. The functioning of the coupled system will be inhibited because the process of electron transfer in photosystem II will be altered. Excess inhibiting compound compared to the amount of plastoquinone will arrest functioning of plastoquinone and culminate in cessation of photosynthesis in the affected plant.