Sometimes it is believed that dead-end operation is significantly efficient. The accumulated particles normally gather on the surface of the membrane during filtration in dead-end processes (Culfaz et al., 2011).
Membrane
Membranes have shown better elimination of 90 percent of diverse ionic species and they offer a high quality permeates in this processes. A single process can be applied to decolourise and eliminate secondary chemical elements that originate from dyehouse liquid waste [25]. From 1990, nanofiltration studies have been conducted with dye bath effluent treatment. The membranes involved are ion selective because of the negatively charged surfaces. Based on the features of a membrane, some elements of secondary chemicals are most likely to filter through the membrane into permeate (Koyuncu and Topacik, 2003).
Many factors such as techniques of dyeing, the contents, the processes and possibilities of recycling influence the choice of size and type of the membrane to be used in a dyehouse [25]. Nanofiltration membranes have also been used subsequent to biological pretreatment of textile liquid wastes. In the main treatment plants, a pilot scale membrane treatment is an ideal choice for a mixture of industrial and domestic effluents. The outcome materials could allow for recycling of permeates in textile plants.
Reactive Dyes
Reactive dye consists of a class of dyes that allow for covalent bond formation with fibre and it generally constitutes its elements after the reaction. This reaction process can be shown as:
Reactive dyes + Fibre = Reactive dye-Fibre (covalent bonding).
Classification of Reactive Dyes
Based on their chemical compositions, reactive dyes are categorised as:
- Chlorotriazine Dyes (MCT)
- Vinyl Sulphone Dyes (VS)
- Heterocyclic Helogen Containing Dyes (HHC)
- Mixed Dyes (MCT-VS)
When temperature is considered as a relevant factor, reactive dyes can be grouped as:
Cold brand reactive dyes
Cold brand reactive dyes are used in low temperature (that is, room temperature). These dyes are extremely reactive with fibre at low temperature.
Hot brand reactive dyes
These dyes work best at medium temperatures of nearly 60ºC. They have medium reactive tendencies with fibre.
High Exhaust brand reactive dyes
These dyes have extremely low reactivity properties with fibres relative to cold and hot categories of dyes. In this process, dyeing takes place at around 80-90ºC.
Properties of reactive dyes
- Reactive dye is anionic in nature
- Reactive dye is a water soluble dye
- They have better wash and light fastness properties
- They have better substantivity
- They form strong co-valent bond with the cellulosic fibre in alkaline condition
- The electrolyte is must for exhaustion of dyes in the fibre
- A certain amount of dyes is hydrolyzed during application (around 15-20%)
- Wide range of colour can be produced with reactive dyes
- Comparatively cheaper in price
Reactive Class of Colours
Reactive colours are the most useful colours for cotton dyeing. These colours can easily form chemical covalent bonds with chemicals in Hydroxyl groups of cellulose fibre. Thus, they are suspended on the substrate and do not rely on weak physical forces for strengths and fastness. The reactive class of colours consists of a wide variety of colours with extremely brilliant hues such as light to exceptionally dark, as well as blacks and Navies with sound unfading qualities and costs. Based on the reactive groups they belong to, such as a triazinyl ring system or vinyl sulphone, a reactive class of colours reacts with the hydroxyl group of cellulose to form neucleophylic substitution or takes part in subsequent reaction (Chinta and VijayKumar, 2013).
Mechanism of Reactive Dye Fixation
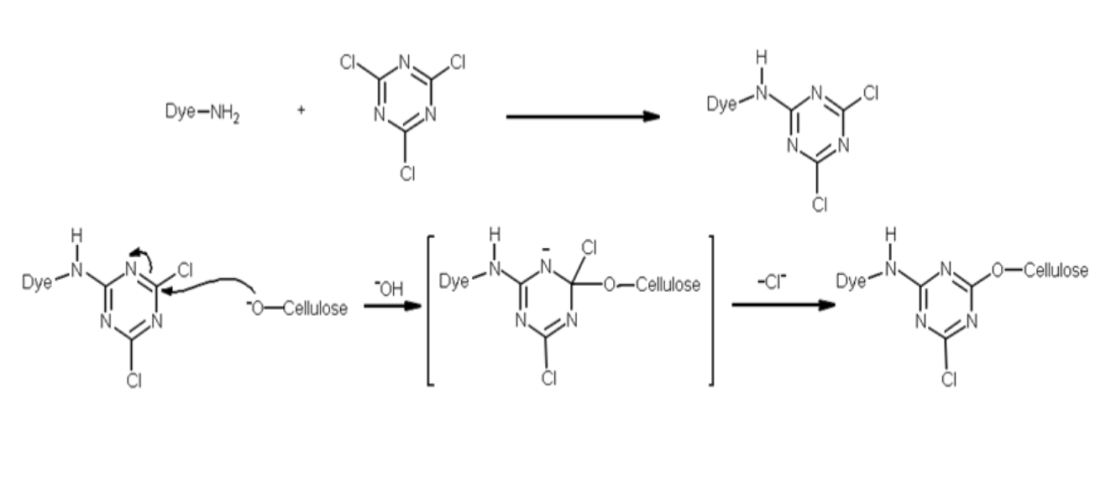
The reaction process involves hydrolysis. Water is the main reactive agent with the dye in this chemical process. However, it is the cellulose fibre, which is involved in the reaction processes because of the following two factors. It overshadows the prevalence of water in the dye bath and results in fixation of the dye to the fibre as the favoured type of reaction.
- There is better attraction between the dye and the chemical compared to water because of the substantivity of the dye and thus after a complete biochemical reaction, the dye on the chemical is far more than that in the bath.
- Cellulose tends to have low dissociation relative to water and creates nearly 25 times greater Cellulose-O – ions over hydroxyl ions.
Covalent Bonding
The ability to use dyes attached to fibres by creating covalent bond has been attractive to dyestuff chemists for a long period because the attachment processes of physical adsorption and mechanical retention have two weaknesses (2) related to either high costs or poor wet fastness. Ahmed (1995) II noted that chemical reaction indicates the creation of a covalent bond between the fibre and the dye. In this regard, two known methods are used to make dye on the fibre and the production process results into a reactive dye towards the fibre.
In 1895, Cross and Bevan produced the first covalently bound mixture of a dye and cellulose [3]. The chemicals used included soda cellulose with benzoyl chloride, which reacted to produce a component of cellulose mono-benzoate and dibenzoate. A reaction of the benzoates with elements of nitric and sulphuric acid formed nitrates. These chemicals then reacted to form aminobenzoyl cellules derivatives. These derivatives could diazotise alongside aromatic amine and phenols to produce colouring chemicals covalently bonded with the cellulose.
Effect of salt on dye aggregation
Both the hydrogen bonds and van der Waals forces facilitate the drawing of different dye particles so that many particles can be found in a mixture because of the gathering of dye particles known as aggregates. These dye aggregates are colloidal electrolytes (Ingamells, 1993). High salt concentrations in dye contents result in low dye solubility. Therefore, in this case, the dissociation equilibrium of acidic dyes takes place, which creates high concentration of non-dissociated dyes in dye houses, which would eventually aggregate. The aggregated dyes formed during this chemical reaction create large volumes and they thus ensure enhanced preservation of dyes.