Relevance of Regulations
Organizations where employees are not regulated, and all interactions between the parties involved are chaotic risk showing negative results. This can be manifested in the loss of reputation, violation of business communication, refusal to follow the established rules.
First of all, it is crucial to recognize the necessity and relevance of regulatory compliance for any organization and medical institution in particular. For this purpose, it is enough to present a company that lacks any signs of orderly work and structuring. Employees are free to come to work at any time and leave whenever they want. Then, scheduled plans are not performed on time, and communication between employees is based solely on the personal and emotional motivation of colleagues. In addition, safety precautions, which are particularly relevant to facilities where employees are at high risk, may not be adhered to due to a reluctance to comply with regulations (Kaire, 2017). Thus, employee negligence may lead to the risk of injury, low productivity and financial success of the company.

Health Care Industry
This problem becomes especially acute when it comes to medical organizations, where the patient’s health is the main result of effective work through compliance with regulations.
While the need to comply with regulations is absolute for companies from all industries, failure to follow established policies in the public or private health care sector can have serious consequences. In particular, it should be understood that a patient’s health condition after hospital discharge is the main product of a health care organization. Therefore, if clinical staff are not prepared to follow regulations and management does not use the main tools of the regulatory compliance program, internal organizational connections are disrupted. Such systemic mistakes can result in inappropriate treatment results, loss of reputation of the organization, and even legal proceedings.
This is why it is so important that medical companies meet the necessary organizational requirements and are able to ensure compliance. In addition to the main goal of providing more systematic and effective care, organizations that already have a culture of compliance receive several significant advantages that ensure competitiveness in the market. The higher the level of compliance, the less likely it is that problems with government agencies will occur. In addition, due to the initiation of audit systems, organizational risks are significantly reduced (Mark, n.d.). In such companies, there is an increase of inter-disciplinary understanding and development and coordination of the general business development strategy. Finally, a regulatory-compliant medical organization traditionally has a higher reputation than its competitors.
Complexed Compliance
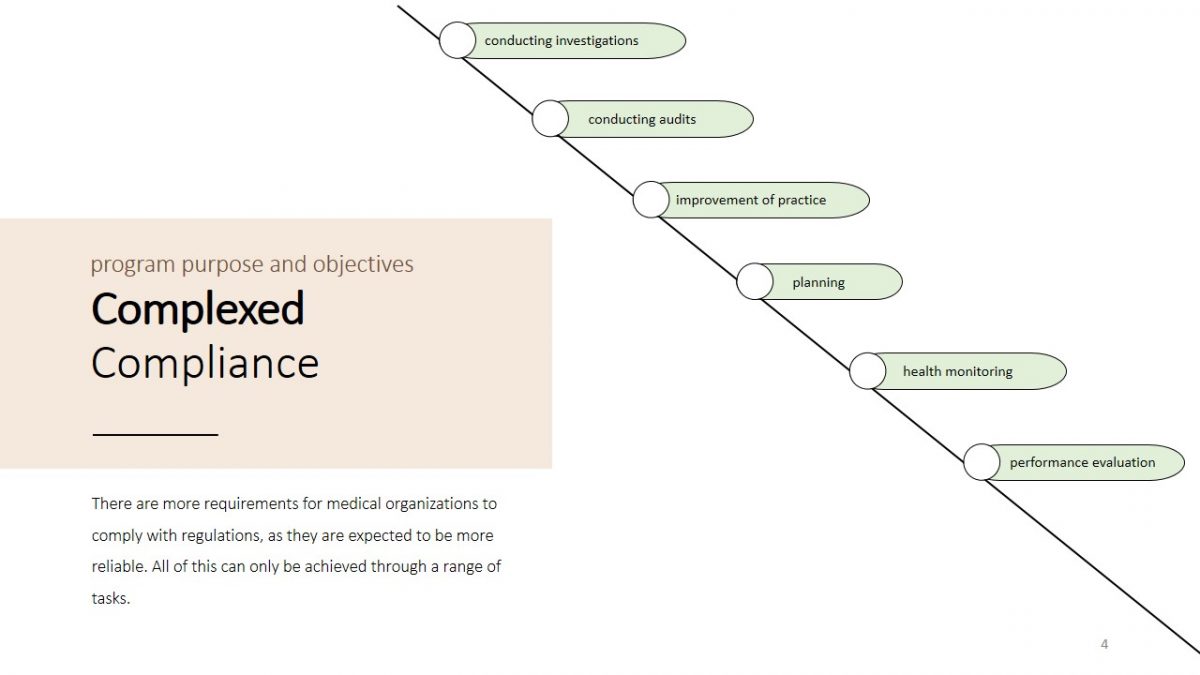
There are more requirements for medical organizations to comply with regulations, as they are expected to be more reliable. All of this can only be achieved through a range of tasks.
- conducting investigations;
- conducting audits;
- improvement of practice;
- planning;
- health monitoring;
- performance evaluation.
There is no doubt that the medical organization is expected to continuously achieve high results and demonstrate the quality that may not be typical of other organizations. To a large extent, this is determined by the task of compliance in the health sector: to achieve and maintain the conditions that are characterized by high patient recovery, compliance with the law, and respect for professional rights of the clinic staff. Such a burden of regulatory compliance puts serious pressure on this area, but it is a prerequisite for ensuring sufficient public health quality. Therefore, in order to facilitate compliance with these regulations, a policy of 7 basic elements can be applied by the management of the clinical organization — or relevant department — to ensure guaranteed compliance.
Seven Basic Compliance Elements
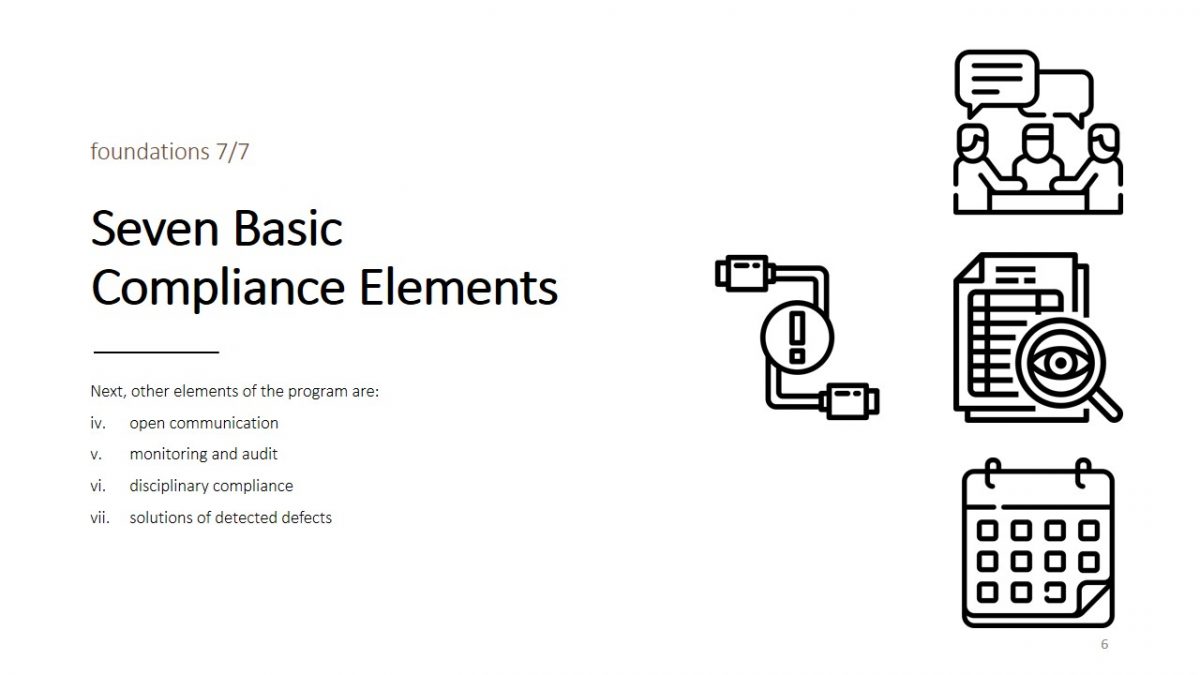
The necessary and sufficient elements include seven factors, including:
- the appointment of an employee or compliance committee;
- employee training and education;
- development of perceived standards and norms;
- open communication;
- monitoring and audit;
- disciplinary compliance;
- solutions of detected defects.
OGI’s guidelines, developed for medical organizations, remain the main document that defines signs of compliance with regulations within a clinical facility. This program covers seven elements at once, each of which is necessary and self-sufficient (Seven elements, 2020). Simultaneously, it should be noted that the implementation of only some of the seven elements is not an indicator of a successful and efficient company but rather indicates the need for further organizational work.
Thus, compliance can be ensured, first of all, by appointing a specialist or compliance committee. This action aims to create a control and accountability environment in the organization, which is necessary to assess compliance. The responsible person will seek to prevent, detect, and correct system vulnerabilities.
The second element is the appropriate training and education. This step requires the organization to conduct training sessions that provide employees with new professional skills and identify the need for compliance. This policy can be aimed at raising the overall awareness of the sample of professional responsibilities and regulations.
A third element of the program is to develop policies and standards that are readily applicable in the workplace. Given the initial complexity of perception of normative documents, the management should pay special attention to the format and structure of the medical institution’s regulations. It is important to ensure that standards and policies are clear to all stakeholders.
In addition, establishing effective communication is also important to improve compliance in the medical organization. Moreover, this implies not only inter-collective communication but also the ways of interaction between the clinic and patients: hotlines, opinion polls, comments. This format of communication facilitates the attempt to detect inconsistencies in the company.
Fifth, monitoring and audit is an important step towards establishing a company that complies with relevant regulations and requirements. The monitoring strategy makes it possible to forecast and plan work tasks and then assess the company’s performance. For example, if a year, the clinic has served fewer patients than predicted, therefore, one can expect damage to the organizational structure at any level. An external audit is also a very successful assessment strategy, as it helps to independently and impartially examine the organization’s culture.
The next element is to ensure that general disciplinary requirements are met. Waiting for all medical organization employees to meet the requirements should be justified by the initial placement of these documents in free and easy access. It is important to ensure that regulations and standards are widely known and consistently followed.
Finally, the last element of the system is to respond to detected shortcomings. This will determine not only the integrity of the clinic system but also the willingness to solve problems and find effective strategies. Managers can address compliance issues, but a more appropriate solution would be to appoint a response team within the Compliance Department. Moreover, periodic audits can become a tool for internal compliance review.

Executing a Compliance Program Involves Risks
It is important to recall that compliance programs are not a voluntary desire of the company to show higher legal and organizational literacy, but the official regulations that oblige institutions to design their own protocols and execution standards. Moreover, such compliance programs can be associated with some risks, especially if the program was not modeled specifically for the given conditions. Effective risk management should, therefore, begin by clarifying the sector in which the company operates and defining the rules that apply to that sector. For example, although cosmetology and plastic surgery are components of medical sciences, different protocols should be used in each work practice.
Five consecutive steps can be performed when the compliance department conducts a risk audit (Zhao, 2019). The first step is to determine the status of current affairs. Once this is done, all potential points of contact between the company and compliance risks should be assessed in detail. The third step is to assess the means and tools to control and prevent the development of organizational threats. Then, it is necessary to evaluate priorities and prioritize actions to eliminate risks. The last step is to update the risk policy periodically.

Compliance Specialist: Role
- Compliance With Requirements:
- Ensuring the company’s compliance with external and internal requirements and standards specific to the health care sector.
- Effective Risk Control:
- Ensuring sufficient control, which measures and effectively manages the existing organizational risks.
- Legal Representation:
- Usually represents the company’s legal entity that provides the obligation to maintain compliance with legal norms and standards.

Fraud, Waste & Abuse
Even the most efficient medical company once has episodes of fraud, waste, or abuse, which have the potential to damage the reputation of the clinic, degrade financial performance and increase the rate of employee reductions. This has to be controlled through compliance programs.
No matter how effective the company is and no matter how thoroughly it checks the work of departments and individual employees: there is no guarantee that sooner or later, episodes of unjustified waste, fraud, or abuse of power will not begin. For example, a nurse director may use their power to pressure and mismanage subordinates, ultimately leading to more layoffs among nurses and a deterioration in the department’s performance. On the other hand, it can be predicted that one day a doctor who prescribes, for example, opioid painkillers for an inpatient patient will decide to take a slightly larger portion of the medicine from the warehouse to keep some of it for themselves. In theory, this not only undermines the credibility of such a specialist but also causes economic damage to the clinic. To sum up, the culture of fraud, destructive behavior, abuse, and waste negatively affects the company in both the short and long term.
Fight Against Fraud
Some elements of compliance management policy can be used to eliminate unwanted effects discussed earlier. This includes developing policies and standards, monitoring and open lines of communication, and rapid intervention.
In particular, as noted earlier, the development and implementation of regulatory requirements contribute to a company’s commitment to compliance and related risks management. The main function of such development is to ensure the correct and stimulating culture of the organization. Then, this element’s relationship with undesirable effects seems transparent: the compliance department needs to create policies that discourage such attitudes and encourage reporting of destructive behavior. Establishing clear rules describing sanctions in case of wrongdoing is a good strategy for this element.
In addition, compliance can be achieved through open communication channels, including hotlines and monitoring. This is particularly relevant for patients who are faced with such behavior by staff. If a nurse or doctor has offered a patient any fraudulent schemes or ways around the law, this should be publicized. This can be done to promote non-acceptance of such situations and to encourage clients and staff to communicate through anonymous channels. This is also due to internal monitoring, during which department employees find non-compliance with regulations and try to inhibit it. For example, if the analysis of reports reveals that medications prescribed by the doctor are consumed faster than usual, there are good reasons for verification.
Finally, if vulnerabilities are found, rapid intervention programs should be used to ensure that the problem stops developing quickly. Such actions could include firing a suspected employee, conducting interviews and training, and conducting legal proceedings.
Proactive vs Reactive
While reactive companies try to respond quickly to emerging problems, proactive ones try to prevent their development even before they appear. Internal audit and whistleblower incentive policies help them do this.
Through internal audit implementation, the company provides an independent assessment of compliance of organizational processes with regulatory requirements and standards. In other words, the company’s management checks the effectiveness and coherence of risk management, corporate governance, and control systems within the organization. In this case, it seems obvious that the company collects necessary material on emerging problems to prevent structural destruction. This approach should be classified as proactive.
However, the policy of encouraging whistleblowers plays a similar role for reactive companies. With valuable and reliable information on violations, the Compliance Department can quickly intervene in business practices and change structure if compliance requires it (Thompson, 2019). For example, if clients of the clinic often complain about the long waiting time in queue, the department should pay attention to this and consider adding a reception desk. In proactive companies, encouraging whistleblowers can be useful if a detected problem has the potential to degenerate into something more, and companies have a chance to eliminate the problem right now.

Medical Data Coding
- Coding medical information is a fundamental technology that ensures effective communication between different departments.
- Lack of proper coding can lead to information exchange disruptions, billing problems, or treatment errors.
- Financial problems are an important part of non-compliance with information coding standards.
- The False Claims Act postulates that anyone who knowingly violated account coding regulations for personal benefit will be punished.
In medical practice, one of the fundamental technologies is the careful coding of information, which determines the possibility of its effective use by various departments. Thus, any inconsistency of opinions and disagreements in reading the material can be neutralized if the information was carefully coded. For example, the doctor’s prescriptions and the diagnosis can be easily interpreted by the nurse if the code has been used.
Based on the above, it seems obvious that inappropriate coding leads to chaotic links within the organization and multiple interpretation errors. In addition, when the encryption is broken, the patient may have trouble establishing an accurate diagnosis and prescribing medications: this can lead to treatment errors and, therefore, to a loss of reputation. If the patient needs an additional procedure performed by a third-party agency, the incorrect coding may lead to inappropriate measurement results (). Thus, financial problems are a significant part of the factors caused by incorrect coding of medical information. There may also be problems with delays in finding a medical record or cases of fraudulent prescription and code changes.
In this context, it is appropriate to mention the False Claims Law, postulating a medical worker’s judicial responsibility who deliberately violated the coding rules for personal benefit. This law, on the one hand, protects the structures of the company against the improper performance of duties and non-compliance with established standards, and on the other hand, it authorizes the illegal activities of doctors or nurses (The False Claims Act, 2020). Specific decisions for which a health care provider can be penalized are the use of more than the required amount of medication, large bills to pay, or the undervaluation of debts to the state. In other words, any action where an employee knowingly violates regulations for personal gain.

The Importance of Proper Documentation
Regardless of the direction of the medical institution, the documentation of all relevant data and stages is the most important rule to achieve mutual understanding between the various departments, as well as to ensure the safety of the organization in the event of illegal legal claims. A well-documented record becomes a guarantee that the probability of abuse and professional misconduct will be reduced. In addition, the patient also benefits from careful documentation, as it gives them clear and articulated instructions.
References
Hayes, A. (2020). Compliance Officer. Web.
The False Claims Act. (2020). Web.
Kaire, M. (2017). Workplace negligence creates dangers for workers and customers. Web.
Mark, J. (n.d.). 4 ways regulatory compliance makes your business better. Web.
Seven elements of an effective compliance program. (2020). Web.
‘Think with your ink’: 4 reasons why proper medical record documentation is vital. (2016). Web.
Thompson, B. (2019). PSA: The new whistleblower policy Australia is enforcing and the consequences for your company. Web.
Zhao, J. (2019). How to conduct a compliance risk assessment: 5 key steps. Web.