General Overview
Although the significance of blood transfusion and the following blood banking process have been acknowledged for quite some time, there has been no uniform approach toward the testing process for the detection of antibodies in erythrocytes up until recently.i The concept of a solid phase test was suggested in the 1990s and is based on the idea of immobilizing one of the agents. After the immobilization phase, the agent in question reacts with the additional reactants that can be located in the liquid phase with the following transfer thereof to the solid phase.
In the early ’90s, the Capture-R solid-phase system was designed to carry out the testing processes successfully. The identified serological testing framework proved to have high sensitivity rates for detecting antibodies in the routine environment. However, the areas of its usage are limited to the anti-IgG-based IAT (indirect algorithm test). Afterward, a solid phase enzyme-linked immunosorbent assay (ELISA) was designed by Biotest, Ltd. to improve the automated reading issue, yet it is currently not viewed as commercially promising. ii
Hypothesis
The application of the solid phase testing process delivers more palatable results than the rest of the tests that are traditionally used to detect the blood type for a blood transfusion because of its high sensitivity rates; therefore, it should be considered as the primary means of carrying out the related tests.
Hemolytic Transfusion Reaction (Acute & Delayed)
Hemolytic Transfusion Reaction (HTR) can be defined as the adverse reaction that a patient develops as a result of the blood transfusion procedure. As a rule, HTR manifests itself in, although it is not restricted to, fever, anemia, urticaria, etc.
The phenomenon of HTR may occur if the patient starts producing antibodies to the red cell antigen that the transfused blood contains yet the patient does not have in their system. Particularly, the absence of the Kidd blood antigen in the patient’s blood and the presence thereof in the blood that has been introduced to the patient’s bloodstream causes the HTR reaction.
HTR manifests itself in two modes, i.e., the acute and the delayed one. In the case of the latter, the negative effects may ensue approximately 12–14 hours after the transfusion. In the instance of an acute HTR, the patient develops resistance to the transfused blood immediately.
Pathophysiology
Acute
As a rule, the following classification is provided for acute HTRs: transfusion-related acute lung injury, or TRALI; bacterial contamination and endotoxemia; nonhemolytic febrile reactions; circulatory (volume) overload; acute hemolytic reactions, and allergic reactions. In TRALI, the human leukocyte antigen and the neutrophil antigens in the patient’s bloodstream react, causing pulmonary edema. The circulatory (volume) overload also causes pulmonary edema since it implies that the coincidental infusion in the patient’s body due to the reaction with the transfused blood should occur.
The presence of bacteria in the transfused blood may cause bacterial contamination in the patient. The cytokines from the leukocytes in the transfused blood, in their turn, cause nonhemolytic febrile transfusion reactions, which trigger fever. Finally, a patient may develop allergic reactions to transfused blood. The allergies may manifest themselves in the form of a rash, pruritus, etc.
Finally, acute hemolytic reactions fall into the categories of immune-mediated and nonimmune-mediated reactions. They are IgE mediated and are represented by rash, pruritus, and similar problems. The reaction is caused by the presence of hypersensitive allogeneic proteins in the patient’s bloodstream.
Delayed
The delayed reactions are typically viewed as life-threatening. The bystander hemolysis phenomenon can be viewed as the key factor in enhancing the DHTR process. As a rule, marked reticulocytopenia is viewed as the primary effect of DTHR, although it is not necessarily present in the patient. Anemia, however, always accompanies the identified health problem, therefore triggering a rapid drop in the patient’s wellbeing.
Antibodies
Acute HTR is typically caused by antibodies such as UgM antiA and antiB. However, it may also be spawned by Rh, Kell, or Duffy. Delayed HTR, in turn, is characterized by the Kidd antibody.
Main Body
It should be borne in mind, however, that the test in question is typically viewed as one of the most complicated procedures of all serologic tests. Therefore, the introduction of the strategies mentioned above served as the premises for building a more efficient approach toward antibody identification. The automated solid-phase antibody screen (SPAS), for instance, was reported as one of the groundbreaking tools in determining the presence of antibodies in the patient’s blood.
Specifically, the test results described by Plapp et al.iii showed that the SPAS testing tool delivered outstandingly accurate results due to high rates of specificity and sensitivity. Furthermore, the study indicated that the emergence of false-positive reactions is quite low—only 2% of the patient’s sera was viewed as the possible source of an error in calculations at an 80% rate of ionic strength reduction.
More importantly, by considering the reactions in question to be non-specific, one would reduce the threat of a false-positive rate to 0.29%. Therefore, the SPAS test was proven to be one of the most efficient tools in locating antibodies in the patient’s blood. The identification of antibodies in the red blood cells of the patient, therefore, occurs at a faster speed once the solid-phase test is applied.
Techniques for Ab’s Detection and Their Comparison
The technological revolution that was experienced globally and the emergence of disruptive technology slowly changed the landscape of the red-cell testing framework. As Knight and de Silva explain, the application of the tube technique, which became a possibility in the mid-40s, truly was a breakthrough that heralded a new era in managing the process of screening for the production of red-cell antibodies.
As a rule, the first tests implied the use of the saline medium; at present, however, the application of the techniques based on the use of albumin and proteolytic enzymes as the means of testing for compatibility has become common practice. One must also give credit to the recent shift toward drawing a line between the tools that permit selecting the antibodies that can be deemed as clinically significant and those that do not. As a result, the ones that do not have any potential effect on the outcomes of the analysis or the identification of changes in the patient’s health status can be dismissed in the course of the analysis.
The given option helps avoid cluttering the analysis with extra information and thus contributes to a faster and more efficient study. Currently, the Indirect Antiglobulin Test (IAT) is used as the primary tool for carrying out the corresponding analysis and locating the presence of antibodies in the red blood cells. When addressing the positive aspects of the testing system, one must mention the fact that IAT provides premises for a much more accurate evaluation and, therefore, a significant increase in the sensitivity of the tool.iv
A more recent study points to the fact that a significant leap has been made in the process of identifying the presence of antibodies in red blood cells. Apart from the solid-phase testing tool described above, the application of the methods, such as a parallel, blinded comparison of tube PEG, as well as the usage of automated methods, has been introduced into the framework. As the comparison made by Bunker, Thomas, and Geyer has shown quite explicitly, the PEG screening can be deemed as the most cost-efficient tool.
While the findings of the study can be deemed somewhat surprising given the previously mentioned properties of the solid-phase testing tool, one must admit that the PEG screening provides the basis for the most sensible use of the available resources while at the same time allowing one to retrieve quite accurate results. It should be noted, though, that the manual SPRCA test delivered results of higher precision compared to PE.
The identified outcome was quite expected because the SPRCA tool as a variation of the manual test helped retrieve a significantly larger number of the false-positive screen test results, as the researchers explain. Furthermore, the fact that the tool under analysis caused the results to become inconclusive indicated that the SPRCA method needs to take a back seat to PEG as a more advanced and accurate testing method.v
Furthermore, the solid-phase approach is often mentioned alongside the strategy known as the tube-low-ionic-strength solution additive indirect antiglobulin test. It should be borne in mind, however, that the recent study on the subject matter considered only the detection of alloantibodies; in other words, the analysis implied the identification of solely the diseases that can be contracted from other people.
At this point, one must bring up the fact that the current information regarding the sensitivity of microtube systems, which can be found in contemporary studies, is rather conflicting and, therefore, cannot provide a proper background for a complete analysis of the tool, as Weisbach et al. explain.
According to the research outcomes, the Capture-R Ready-Screen tool allowed for retrieving the results of the highest sensitivity rate, which meant that it is significantly more effective than the manual reading framework. The latter showed a rather high rate of data omission in the process, therefore reducing the accuracy levels and causing the research process to become convoluted: “By automated reading of Solidscreen II results, four weak-positive reactions detectable by manual reading were missed (two anti-Jka, one anti-D, and one antiCD).”vi
Another addition to the general framework of managing the red blood cell antigen testing,vii the use of the automated gel column should be added to the discussion. The study results showed quite graphically that the use of the gel column agglutination method can be deemed as the most efficient in locating the antibodies in the patient’s red blood cells. The fact that the identified approach is also cost-efficient and, therefore, permits the most adequate use of available resources of all tools considered in the study also deserves to be mentioned. The cost issue seems to be the definitive factor that determines the sensibility of applying the studied tool to the actual clinical environment. The quality issue also needs to be listed among the primary factors that define the contemporary framework of the antibody test design:
Gel column agglutination has unique advantages in detecting mixed-field agglutination patterns. The gel column agglutination method can utilize inert buffered gel cards or ABO/D type-specific gel cards. For inert buffered cards, the patient plasma and reagent RBCs are incubated together followed by centrifugation through a gel matrix. Indeed, a closer look at the information provided in the studies shows that the gel method allowed for a precise location of the issue in all four of the cases.
Specifically, a mixed-field reaction could be observed in Patients A, B, C, and D alike after the gel method was used and the corresponding analysis tools were applied. Differently put, a strong propensity for the gel column agglutination technology to deliver the results of high accuracy and veracity can be located. The manual and automated techniques used in the research, although also delivering rather accurate results, proved to be of a rather low cost-efficiency. In other words, the cost of the tools in question and the positive outcomes that they deliver does not correlate in contrast to the gel coagulation technique mentioned above.
In addition to the opportunities for saving time and money that the gel method implies, it also serves as the means of arranging the analysis-related processes under the categories of manual and computer-regulated ones. Particularly, the automated gel column agglutination process is carried out as a fully automated process, whereas the ABO grouping and the D typing processes are performed manually. The differentiation between the approaches toward the data analysis also makes the device look rather favorable compared to other approaches, including the solid-phase method and the direct agglutination microwell framework.viii
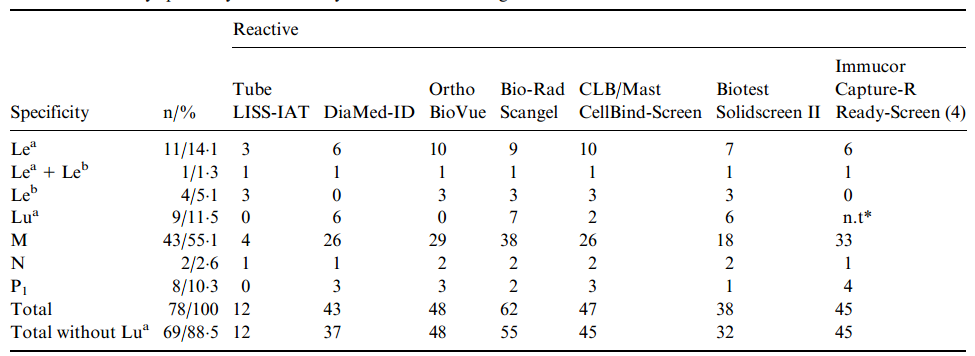
Table 1. Antibodies Obtained and Detected with SPAS and Agglutination
At this point, the issue of automation in the identified process needs to be brought up. According to Bajpal, Kaur, and Gupta, the enhancement of automation as part and parcel of the immunohematology processes is bound to lead to a significant rise in the healthcare service quality. Specifically, the authors address the fact that the three approaches to the detection of antigens in red blood cells are typically deemed as the primary devices for promoting automation in the blood transfusion area. The choice between the semi-automated and the fully automated devices has not been made yet; however, the decision hinges on the workload and the budget of the facility to a considerable extent.ix
Conclusion
The process of blood transfusion is often fraught with the development of hemolytic transfusion reactions. Primarily, the problem regarding the percentage of errors emerging in the testing process needs to be addressed. Once the issues concerning the cost and the accuracy of the devices are addressed, the quality of healthcare can be increased significantly. Particularly, the process of blood transfusion will occur in the environment that is bound to lead to success in blood transfusion and the related procedures. To be more exact, the detection of antibodies in the patient’s red blood cells will become a much simpler process with the adoption of the techniques mentioned above.
Reference List
- Ching E. Solid Phase Red Cell Adherence Assay: A tubeless method for pretransfusion testing and other applications in transfusion science. Transfusion and Apheresis Science. 2012; 46(3):287-291.
- Duguid J K M, Bromilov I M. New technology in hospital blood banking. Journal of Clinical Pathology. 1993; 46(7): 585-8.
- Plapp F V, Sinor L T, Rachel J M, Beck M L, Films M I, Coenen W M, et al. A solid-phase antibody screen. Brief Scientific Reports 1984; 82(6): 719-21.
- Knight R C, de Silva, M. New technologies for red-cell serology. Blood Reviews. 1996; 10(2): 101-110.
- Bunker M L, Thomas C L, Geyer S J. Optimizing pretransfusion antibody detection and identification: a parallel, blinded comparison of tube PEG, solid-phase, and automated methods. Immunohematology. 2001; 41(10): 621-626.
- Weisbach V, Kohnhauser T, Zimmermann R, Ringwald J, Strasser E, Zingsem J, Eckstein R. Comparison of the performance of microtube column systems and solid-phase systems and the tube low-ionic-strength solution additive indirect antiglobulin test in the detection of red cell alloantibodies. Transfusion Medicine. 2006; 16: 276-284.
- Adriaansen M, Perry H. Validation of column agglutination technology for blood group alloantibody titration. New Zealand Journal of Medical Laboratory Science. 2013; 3: 92-96.
- Summers T, Johnson V, Stephan J P, Johnson G J, Leonard G. The value of automated gel column agglutination technology in the identification of true inherent D blood types in massively transfused patients. Transfusion. 2009; 49(6): 1672-1677.
- Bajpal M, Kaur R, Gupta E. Automation in immunohematology. Asian Journal of Transfusion Science. 2012; 6(2): 140-144.