A potential application for the Blatter radical (1,3-diphenyl-1,4-dihydro-1,2,4-benzotriazin-4-yl) includes signal amplifier additive, polymerization initiator, photodetector and conductor, ligand, pH sensor, and molecular electronic as well as organic radical battery. A multistep approach was taken to prepare the Blatter radical for further investigation. Benzoyl-2-phenylhydrazine was used as an intermediate in the synthetic route design, along with N-phenylbenzenecarbohydrazonoyl chloride, (Z)-N N’-diphenylbenzohydrazonamide, and N-phenylbenzenecarbohydrazonoyl chloride. The compound N-phenylbenzenecarbohydrazonoyl chloride has been isolated up to this point.
Introduction
The Blatter radical, also known as the 1,3-diphenyl-1,4-dihydro-1,2,4-benzotriazin-4-yl radical, was discovered in 1968 and was named after the scientist who discovered it. It is highly regarded for its exceptional stability and its one-of-a-kind magnetic characteristics. Such properties can be used in various applications, including signal amplification in NMR and molecular electronics, among others. Beyond the numerous applications for which the radical is suitable, it can be used as a building block for various functional products. In nature, radicals are complex, and the synthesis of radicals is no different.
For this article, we will look at how to synthesize the Blatter radical in multiple steps and determine its spectral characteristics.
Magnetic Properties
- There are two known magnetic properties for the Blatter radical’s derivatives: 3-tert-butyl-1-phenyl-1,2,4-benzotriazinyl and 1-(4-chlorophenyl)-3-phenyl-1,2,4-benzotriazinyl Measurements of magnetic susceptibility reveal the presence of weak antiferromagnetic interactions between the radical centers.
- Between 10K and 300K, these Blatter radical derivatives exhibit Curie-Weiss behavior, consistent with ferromagnetic interactions between S-1/2 radicals and C-0.376nemu-K and 0.3 K. When the temperature falls below ten thousand degrees, the compounds lose their Curie-Weiss behavior. The increase in XT following radical cooling demonstrates the presence of ferromagnetic interactions between the radical’s spins.
- A one-dimensional Helsenberg ferromagnetic chain model can be used to interpret the magnetic behavior of molecules arranged in a close pi-stacked configuration.
Scheme
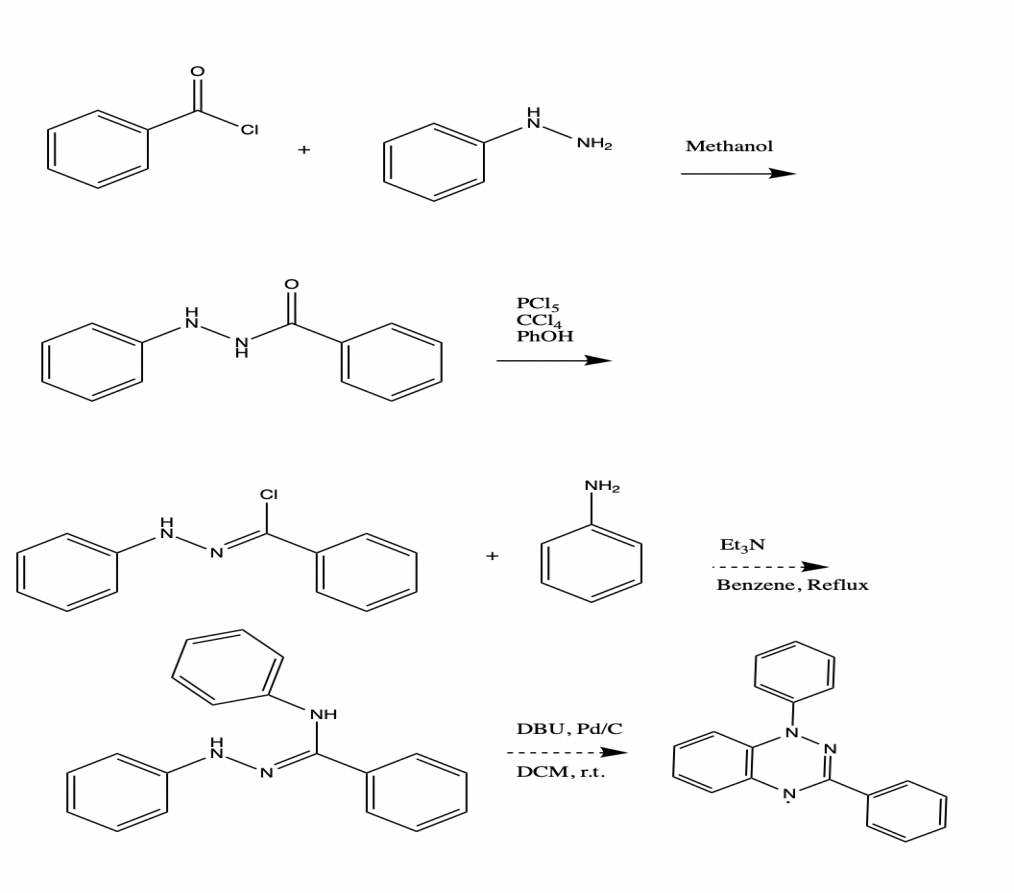
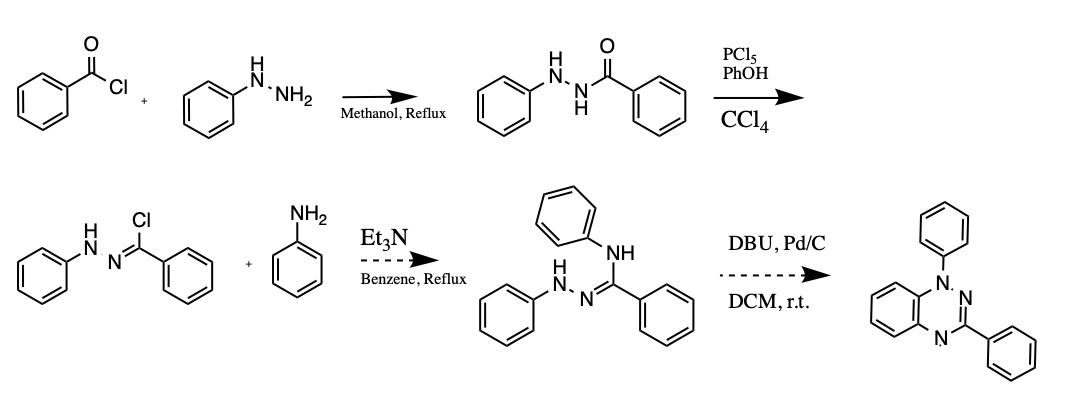
Materials
Benzoyl Phenylhydrazine
- Phenylhydrazine
- Pyridine
- Benzoyl chloride
Hydrazonyl Chloride
- Benzoyl phenyl hydrazide
- Carbon tetrachloride
- Phenol
- Phosphorus pentachloride
- Methanol
Method for benzoyl phenyl hydrazide
The methodological basis of the present study is the organic synthesis of hydrazonyl chloride followed by spectroscopy procedures. Spectroscopic studies were used to determine the purity of the synthesis, so this was used as an examination of organic synthesis. Step one to produce benzoyl phenyl hydrazide, 20ml pyradine was added slowly to a mixture of a 20 mmol (2.16g) of phenyl hydrazide and 20.3 mmol (2.8g) of benzyl chloride were used in an ice bath for 20 minutes. After removing the mixture from the ice bath, the preparation was cooled for fifteen minutes at room temperature. The next step was the use of filtration with dilution of distilled water – the precipitate was crystallized and MeOH was added.
Measurements
From our calculations theoretical yield was at 2ml which is equivalent to 2.2gr or 20.3mmol %yield = (actual yield (Sample without weighing boat)/theoretical yield) x 100.
Table 1: First Trial
Table 2: Second Trial
Table 3: Third Trial
Table 4: Fourth Trial
Table 5: Fifth Trial
Table 6: Sixth Trial
Table 7: Seventh Trial
AVERAGE % Yield=23.36 %
Method for Hydrazonyl Chloride
Step two to produce hydrazonyl chloride, 0.0023mol (0.495g) of benzoyl phenyl hydrazide was dissolved in 7ml of carbon tetrachloride and 0.0026mol (0.535g) of PCl5 was added. Reverse boiling for three hours was used, after which the solution was cooled in an ice bath to reach 0 degree Celsius. To the cooled solution, 1.16g of phenol was dissolved in 3.5ml of CCl4 and added drop by drop to the cooled solution. The solvent was evaporated using a rotary evaporator, after the liquid evaporated and solid formed, methanol was added to the dried solid to dissolve. The product was placed in the fridge to form crystals. These crystals were used for subsequent spectroscopic studies.
Note: A new Procedure for step two was performed, but it did not work, the producer is as follow:
- 1.50g of benzoylphenyl
- hydrazine is mixed with 0.57ml of aniline and a 1.20ml of TEA in a 25ml benzene
- The reaction was then set to reflux overnight, then it was dried in the rotavap
- 50m of cold water is added
Measurements
In this case my theoretical yield was 0.5416 grams.
%yield = (actual yield (Sample without weighing boat)/theoretical yield) x 100.
Table 1: First Trial
Table 1: Second Trial
Table 1: Third Trial
Table 1: Fourth Trial
Note: Fifth trial didn’t form any crystals.
AVERAGE % Yield= 11.67%
Results and discussion
The authors of the current study devised an experimental scheme for producing Hydrazonyl Chloride by employing a multistep approach. Step one involved the synthesis of Alizadch, and Moafi (2016) proposed creating benzoyl phenylhydrazied as a second step, which was accomplished in step two. While the current study’s goal was to synthesize the substance, it also sought to analyze it using a variety of spectroscopies, which was the primary objective of the previous research. These tests include the examination of ultraviolet and visible spectra and nuclear magnetic resonance on hydrogen and carbon nuclei, among others. To achieve the desired result in step one of the syntheses, I slowly added a 20ml solution of pyradine to a mixture of 20mmol (2.16g) of phenyl hydrazide and 20.3mmol (2.8g) of benzyl chloride in an ice bath for 20 minutes to achieve the desired result.
The theoretical yield at 50 percent is 2.48g (2.8g+2.16g=4.96g), and the actual yield is 2.48g. Our calculations showed that the theoretical yield of the reaction was 50 percent and that the practical yield of the reaction was 20.75 percent, which is in line with Alizadch and Moafi (2016)’s findings. In step two, the article “Synthesis of the Hydrazonyl Chloride” was used as a reference. It was published in the journal synthesis. Using an experimental scheme to produce Hydrazonyl Chloride per the description found in the article for this study, I carried out the current work. The solution was boiled explicitly in reverse for three hours, after which the product was cooled in an ice bath to achieve an internal temperature of zero degrees Celsius. It was necessary to dissolve 1.16g of phenol in 3.5 mL of CCl4 before adding the solution drop by drop to a cooling solution until the solution was cooled entirely. Notably, there was a statistically significant difference in yields between the different experiments. For our analysis, we used an average yield.
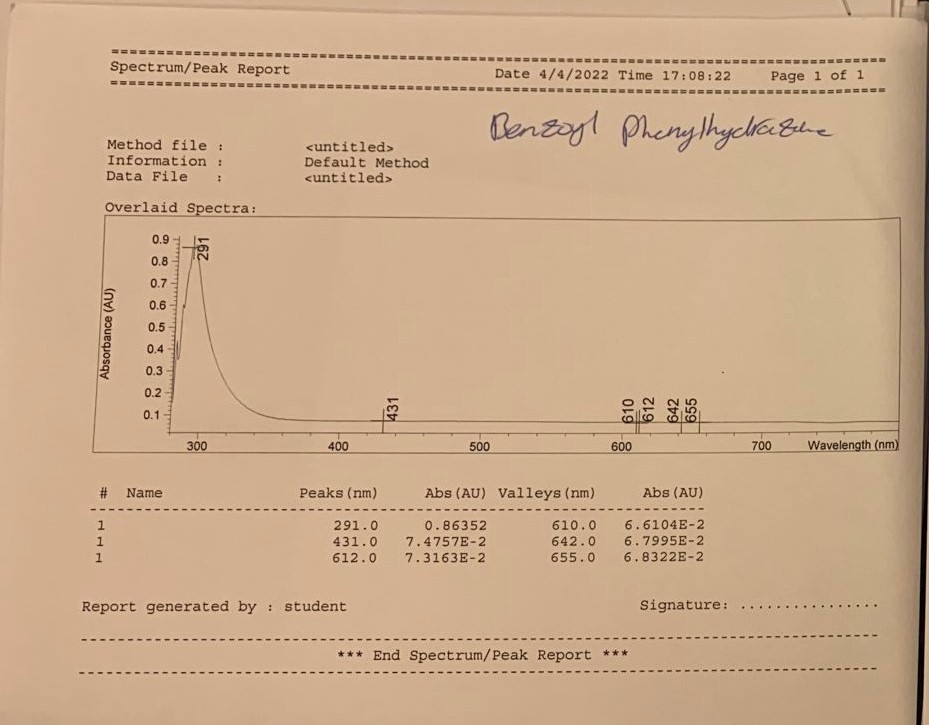
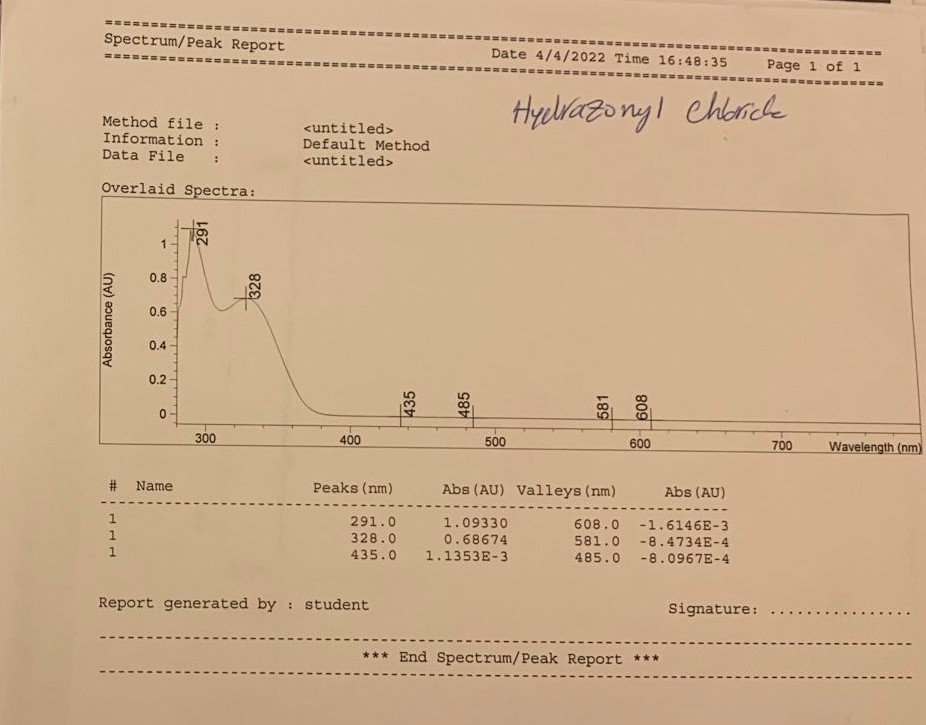
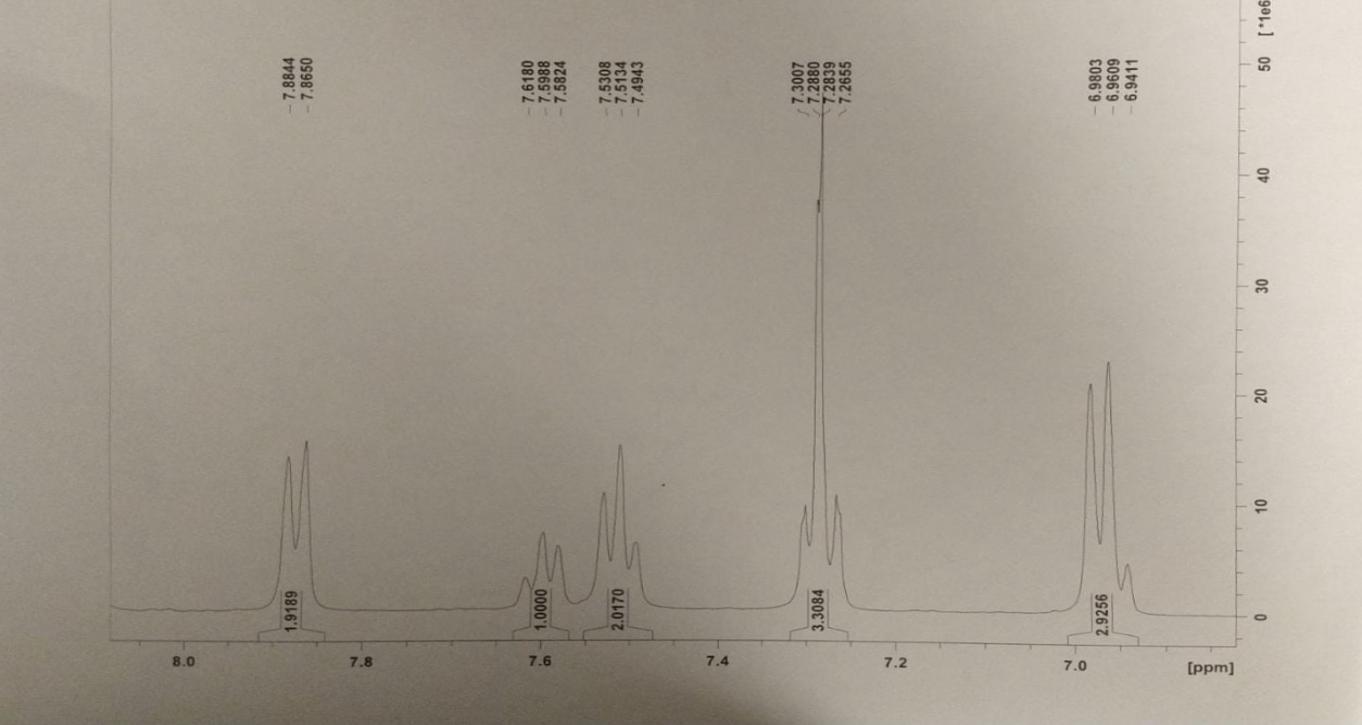
Conclusion
In the present study, the organic synthesis of hydrazonyl chloride was carried out, followed by spectroscopy procedures, and then subjected to laboratory testing per the experimental scheme outlined in the article by the other authors. The results of the laboratory testing were published in the journal. In the case of hydrogen nuclei, ultraviolet and nuclear magnetic resonance spectrometry revealed the presence of the expected characteristic bands, demonstrating that we had obtained the intended substance.
Experimental Data
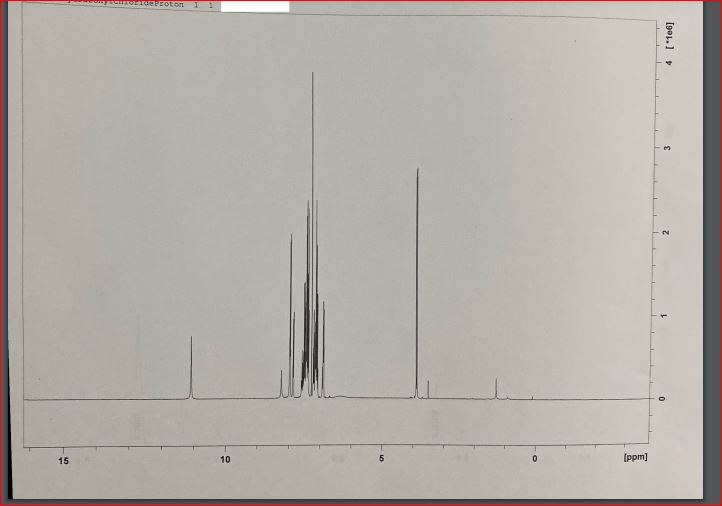
The resulting compound was subjected to various spectrometry procedures, and the qualitative outcomes of the present experiment were represented by the multiple types of spectrograms obtained for the two steps. According to the results of the UV-visible spectroscopy, a strong peak at 291 nm (for both steps – this confirms the presence of a bathochromic shift due to the conjugation of benzene rings within both products) was observed (Nishide et al., 1993). A characteristic absorbance difference was observed at the 0.85 Au and 1.1AU concentrations for steps one and two, respectively. This method can detect evidence of an * transition in the -N=N- bonds within the molecule’s structure (Cho et al., 2017).
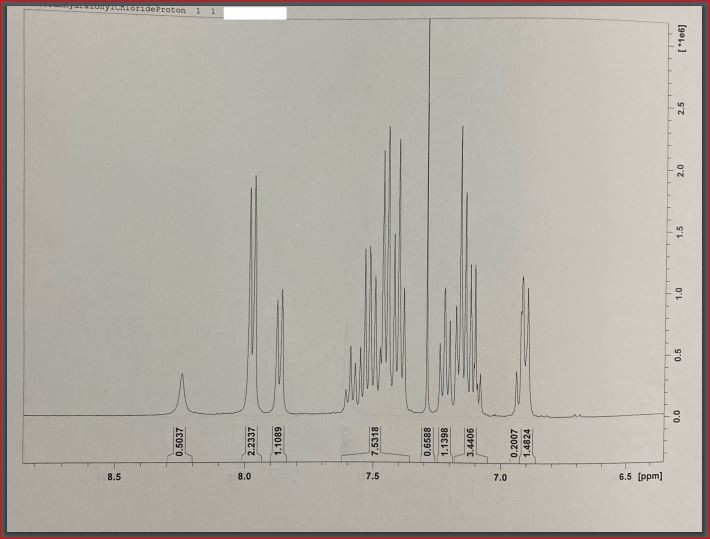
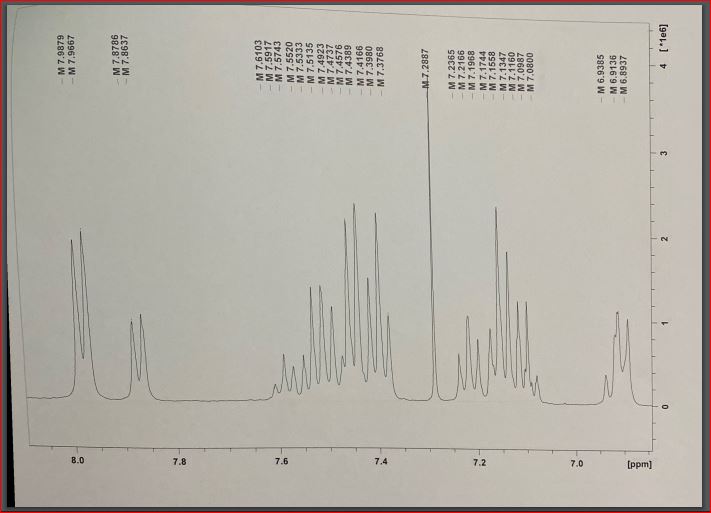
Finally, four attempts at proton nuclear magnetic resonance for hydrazonyl chloride were made, with several apparent peaks observed in each effort. An increase in the peak value at 7.4 indicated the presence and strength of hydrogen bonding between an essential oil ring and another ring (Starkey, 2022). On the 8.0 (2.2337) ppm bands, which correspond to hydrogen associated with aromatic rings, a mixture of peaks could be seen, which was unexpected. Higher energy is found in the downfield region of the spectrum, on the left side of the range (higher ppm). A higher field of force means lower power – the right side of the content (lower ppm)
References
Alizadeh, A., & Moafi, L. (2016). Cheminform abstract: Convenient one-pot synthesis of spirooxindole derivatives containing a 1,3,4-thiadiazine scaffold. ChemInform, 47(49). Web.
Cho, E. N., Zhitomirsky, D., Han, G. G., Liu, Y., & Grossman, J. C. (2017). Molecularly engineered azobenzene derivatives for high energy density solid-state solar thermal fuels. ACS Applied Materials & Interfaces, 9(10), 8679-8687.
M-(3,3-Dimethylureido)phenyl-tert-butyl carbamate 4849-32-5. (2012). Sax’s Dangerous Properties of Industrial Materials. Web.
Nishide, H., Kaneko, T., Yoshioka, N., Akiyama, H., Igarashi, M., & Tsuchida, E. (1993). Poly[[4-(n-tert-butyl-n-hydroxyamino)phenyl]acetylene] and the magnetic property of its radical derivative. Macromolecules, 26(17), 4567–4571. Web.
Kapurwan, S. (2021). Halo-substituted benzotriazinyl radicals and its metal complexes: Synthesis, structures and magnetic properties. Web.