Introduction
Dysport (also known as Reloxin) is a derivative of the toxin botulinum, secreted by the bacteria Clostridia botulinum. Before it found application in therapeutic surgery, the drug was previously used to treat muscle spasticity, which is a movement inhibiting condition (Hayman et al 2000). Presently, however, Dysport, which is marketed by Medicis, is an anti-wrinkling agent that is used to reverse the effects of eye lines, facial wrinkles, and other body muscles. In cosmetic surgery, it is used to cure ‘dynamic wrinkles,’ which includes “those lines across the forehead (horizontal rhytids), around the eyes (crow’s feet), and between the eyebrows (glabella)” (Steiger 2010). The drug works by treating facial muscles that specifically cause wrinkles, which removes the dynamic muscles, leading to an improvement of facial appearance.
Dysport is derived from the DNA structure of the abobotulinum toxin, which acts as the chemodenervation (weakening) agent that inhibits the progress of dynamic muscles. It works similarly to Botox, only that the former spreads faster and affects a wider area in a relatively short period, compared with the latter which takes much more time to diffuse (Steiger 2010). However, this does not presuppose that one is better than the other. On the contrary, the exhibited differences point to variations of dosage and administration, with the ratio between Dysport being 3: 1 (Jost, 2003, 38). Both drugs are essentially the same since they belong to the botulinum toxin type A products.
Nonetheless, they are not entirely equivalent due to their difference in strengths. For a product to be considered a generic of another brand, both need to have the same dosage and therapeutic results by over 90 percent, which is hardly the case for Dysport and Botox. Similarly, in the case of generically equivalent variants of the same drug, there are usually no precautionary measures taken when changing prescriptions from one drug to another. In considering the two versions of botulinum toxin, their chemical composition is significantly different in terms of concentration, with Dysport estimated to be three times higher than Botox. Some research studies have indicated a prominence of an even bigger ratio difference, with one suggesting that “the strength of BOTOX to Dysport is estimated to be about 2 to 4, so they cannot be used in similar doses, and while Dysport diffuses more widely than BOTOX, it means that treating one section of the face with Dysport will have a wider effect than treating the same portion with BOTOX” (Steinsapir 2010). Thus, the difference in their composition is reflected in the different ways their effects are manifested. It is on these grounds that Dysport is regarded to be unique in its application in cosmetic surgery.
Pre-formulation
The physiological effects of botulinum toxin were first described in the 1820s, and since then specific formulations have been developed for “botulinum neurotoxin type A (BoNT-A), and a new one for botulinum neurotoxin type A formulation (BoNTA-ABO; and Dysport [abobotulinumtoxinA]” (Pickett, 2009). The study on the science behind Botulinum Neurotoxin A-ABO in clinical use says that for over twenty years, the formulations have been used mostly in Europe by various companies “under the brand name Dysport (Clostridium botulinum type A toxin–hemagglutinin complex; by Ipsen Biopharm, Wrexham, UK)” (Pickett, 2009).
Symptoms treated by Dysport
Cervical Dystonia
The medicinal properties of Dysport, most of which it shares with Botox includes the treatment of cervical dystonia, where it “inhibits the release of the neurotransmitter, acetylcholine, from peripheral cholinergic nerve endings, to reduce the severity of abnormal head position and neck pain in both toxin-naïve and previously treated patients” (Medicis Aesthetics Inc 2010) and in patients with Parkinson’s disease (Tuite and Fernandez 2009, 164). In adults aged over sixty-five years, the drug temporarily smoothes facial features by improving the appearance of glabellar lines which are commonly associated with corrugator and procerus muscle formation activities. When unchecked, these (dynamic) muscles pull the skin’s tendons leading to the formation of skin wrinkles on the face and under the eyes. It can also treat tongue dystonia, which is the abnormal protruding of the tongue (Barnes and Ward, 2007, 117).
Dysport has also previously been used to treat patients with multiple sclerosis and hip disabling spasticity (a medical condition in which tendon reflexive response is inhibited, causing resistance in movement). In this case, Dysport is used to “prevent the release of acetylcholine at neuromuscular junctions, thereby inhibiting muscle contraction” (Hayman et al 2000).
Dosage: the prescription for Dysport is not substitutable with other products derived from botulinum toxin: “care is needed to prevent potentially hazardous mistakes when prescribing, particularly if switching preparations” (Stevenson and Thompson, 2006, 75). This is because “its units of biological activity are not comparable with or convertible into units of any other botulinum toxin products” (Medicis 2010). The manufacturers recommend the treatment dosage for cervical dystonia as 500 units, administered to each affected muscle, for a period of two to three weeks. If the need arises for dose modification, adjustments of 250 unit steps can be made considering the responses of individual patients. Re-treatments are carried out on a 12-week cycle, based on progressive symptoms. However, no dosages have been researched for the treatment of children under the age of 18.
Preparation of Dysport dosage for cervical dystonia is on a single-use vial. The drug’s marketer, Medicis, indicates that 500 units of a vial are reconstituted in a 1 ml solution containing Sodium Chloride at a concentration of 0.9%. The saline should not have any traces of preservatives, and the resultant solution should yield 500 units in each ml. then “Each 300 Unit vial of Dysport is to be reconstituted with 0.6 mL of 0.9% Sodium Chloride Injection USP (without preservative) to yield a solution equivalent to 250 Units per 0.5 mL” (Medicis 2010). The end solution is usually clear and colorless, without any solid matter. If not, then it should not be administered. In administering the drug, a 23/25 gauge needle is used, four hours after reconstituting the drug, during which period it is refrigerated at 2–8°C, and free from ultraviolet rays (Benedetto, 2006 237).
Rheological Properties
Dysport is less viscous, as it can diffuse more easily into the skin tissues. This property is responsible for its ability to produce “Muscular activation by elicitation of the tonic stretch reflex on skin tissues, and to achieve a positive action on the rheological properties of spastic muscles even in the short term” (Baricich, et al, 2008, 872). Perhaps it is due to its high rheology which makes Dysport diffuse into the tissues faster than Botox.
In the treatment of glabellar lines, a 50 unit dose is administered in five even aliquots, each consisting of 10 units. In adults, the effects could last for as long as four months, and therefore the duration of administration should not be shorter than three months. In addition, re-treatment procedures should follow previous ones. Dysport treatment for glabellar is not allowed for patients below 18 years of age.
The preparation of the dose is also for a single vial dosage. 300 units vial of Dysport is reconstituted in a 2.5 ml of 0.9 percent saline solution free of preservatives (Alam et al, 2008, 37). The resultant solution is a concentration of 10 units for every 0.08 ml, which is administered in portions of five equal dosages, using a 30 gauge needle.
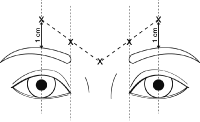
The injection procedures should start with identifying the corrugators and procerus muscles responsible for glabellar wrinkle lines. This is done by asking the patient to frown, and then palpitating over the rigid muscles. In cases of corrugator and atrophic muscles, the skin exhibits furrowed marks which resemble furrowed and vertical skin wrinkles, similar to the furrowing of muscles when one frowns.
When injecting the affected areas, care should be taken to avoid injecting the toxin beyond 1cm above the eyebrow. This may result in allergic reactions by the eye muscles and cause drooping of the eyebrows. To give an injection, the needle is “advanced through the skin into the underlying muscle while applying finger pressure on the superior medial orbital rim” (Medicis, 2010). This is done using a needle of 30 gauge to introduce five doses of 10 units each, one injection to four different points on either side of the affected muscle. The last one is injected into the procerus muscle as the figure below shows.
Cases in which dysport may be prohibited involve allergic reactions to the drug (and any botulinum toxins) or any of its constituent components. Similarly, it should not be administered if the sites for the injection are infected.
As noted before, dysport is highly diffusive and consequently, its effects cold spread beyond the targeted sites. Symptoms for this reaction are similar to the action the toxin botulinum, which include “asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties” (Medicis, 2010). If the toxin is ingested, it can lead to difficulties in swallowing and breathing, and sometimes death.
The risk of contamination is more pronounced in children with spasticity undergoing treatment. In addition, patients with prior complications in breathing and swallowing coul also experience these symptoms, due to the weakening effect of the drug on muscles associated with breathing and swallowing. This may lead to further infection of other respiratory muscles. Similar symptoms, albeit significantly mild, may also be observed in patients being treated for cervical dystonia. This could be caused by the adverse effects of botulinum toxins, when they infect and weaken those neck muscles responsible for ventilation. Such an occurrence may cause serious breathing complications, especially in patients with respiratory problems. The precautionary measure is to avoid administration of the drug to patients with previous or current respiratory disorders.
In the case of cosmetic surgery, administration of Dysport may cause muscular atrophy (excessive muscle weakness) and lead to “marked facial asymmetry, inflammation at the injection site(s), ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin or the inability to substantially lessen glabellar lines by physically spreading them apart” (Medicis 2010).
Nonetheless, the administration and effectiveness of dysport is mired with the same complications associated with its competitor version, Botox. Neither is absolutely risk –free as both are purified versions of botulinum — a nerve toxic substance secreted by bacteria and has the potential to cause botulism, ‘where injections can cause droopy eyelids or uneven eyebrow” (Jejurikar 2010) and other eye side effects (Maio and Rzany, 2007, 8). Though health risks are rare, they are life threatening when they occur.
Conclusion
In conclusion, the drug dysport is a derivative of the toxin botulinum, produced by the bacteria clostridia botulinum. It is used for therapeutic purposes especially in cosmetic surgery where it inhibits the spread of dynamic muscles, which are known to cause facial wrinkles and eye lines. Before its recent application in cosmetic surgery, it has been used previously to treat muscle spasticity, which is a movement inhibiting condition. It is also used in treating related conditions such as cervical dystonia. Its applications in medicine are similar to those of Botox, although they have different procedures of prescription. While Botox uses 100 units vial, Dysport is given in 500 units, with 2.5 mL and 4 ml volumes respectively (Lipham, 2007, 29, Brin et al 2004, 34). However, both are highly toxic and could lead to health risks in cases of contamination. Thus, the significance of either is not pinned to their composition or extent of manifested effects, but in terms of their safety and long term implications.
References
Alam, M., Gladstone, H. B., Tung, r. c., 2008. Cosmetic Dermatology. Elsevier Health Sciences, New York.
Baricich, A. et al 2008. A single blinded, randomized pilot study of botulinum toxin type A combined with non-pharmacological treatment for spastic foot. Journal of Rehabilitative Medicine, Vol. 40 New York: Foundation of Rehabilitation Information, 870-872.
Benedetto, A. 2006. Botulinum Toxin in Clinical Dermatology. New York: Taylor & Francis.
Brin, M. F. et al, 2004. Dystonia: etiology, clinical features, and treatment, CRS Press, New York:
Hayman, N., Barnes, M., Bhakta, B., et al, 2000. Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective randomized, double blind, placebo controlled, dose ranging study. Journal of Neurology, Neurosurgery& Psychiatry, Vol. 68 707-712. Web.
Jost, W. H., 2003. Botulinum toxin in painful diseases. New York: Karger Publishers.
Lipham, W. J. 2007. Cosmetic and Clinical Applications of Botox and Dermal Fillers. New York: SLACK Incorporated.
Maio, M., Rzany, B., 2007, Botulinum Toxin in Aesthetic Medicine. Springer, New York.
Medicis Aesthetics Inc., 2010. Medication Guide Dysport. Web.
Singer, N., March 11, 2010. The Dysport Challenge to Botox. The New York Times, New York.
Steinsapir, K., 2010. Is Dysport Better Than Botox? Ezine Articles. Web.
Steiger J., 2010. What is Dysport? Web.
Tuite, P. J., Fernandez, M. D., 2009. Parkinson’s Disease: A Guide to Patient Care. Springer Publishing Company, New York
Stevenson, V., Thompson, A. J., 2006. Spasticity Management: A Practical Multidisciplinary Guide. CRC Press New York
Ward, A. B., Barnes, M. P., 2007. Clinical uses of botulinum toxins. London: Cambridge University Press
Wortzman, M. S., Pickett, A. 2009. The Science and Manufacturing Behind Botulinum Neurotoxin Type A-ABO in Clinical Use, Aesthetics Surgery Journal, American Society for Aesthetic Plastic Surgery, New York