Introduction
The experiment established the effect of various liquids (coffee and salt) on urinary excretion. Further, the results obtained were compared with the work of the renal system when consuming plain water. The experiment used concepts such as fluid balance and renal physiology. The fluid balance is the ratio between the amount of fluid that has entered the body and the amount removed from it over the same period. The value of fluid balance is due to the fact that a violation of the water balance leads to serious health problems (Masuda et al., 2020). For example, a breach of the fluid balance affects the concentration of attention and the ability of the brain to solve logical problems.
Renal physiology is kidney functions; the importance of it in the body is due to the excretory function of the kidneys. They remove decay products, excess water, salts, harmful substances, and some medications. Therefore, renal physiology plays a unique role in maintaining the body’s normal functioning. Components such as countercurrent mechanism and mass balance are essential in renal physiology (Wall et al., 2020). The countercurrent mechanism is the movement of the tubular fluid in the descending and ascending sections of the Henle loop. Mass balance is the regulation of water and electrolyte balance by the kidneys.
The ascending knee of the Henle loop plays a decisive role in the operation of the countercurrent mechanism. The significance of this organ is due to the ability of the epithelium to actively reabsorb sodium ions into the surrounding interstitial space, maintaining the hyperosmolarity of the interstitial fluid. Under the influence of the countercurrent mechanism, functions essential for urine concentration are also implemented.
An equally important component in renal physiology is the mass balance of water and electrolyte levels. To maintain homeostasis, the release of water and electrolytes must precisely match their intake. If the information exceeds the discharge, the amount of this substance in the body will increase. If there is less of the sense than is excreted, then its amount will decrease (Dalal et al., 2021). Therefore, the experiment’s objectives are experimental confirmations of the excretory function of the kidneys. The experimental hypothesis directly depends on the need to maintain the mass balance of water and electrolyte levels and the activity of renal physiology.
Results
Graphing the Result
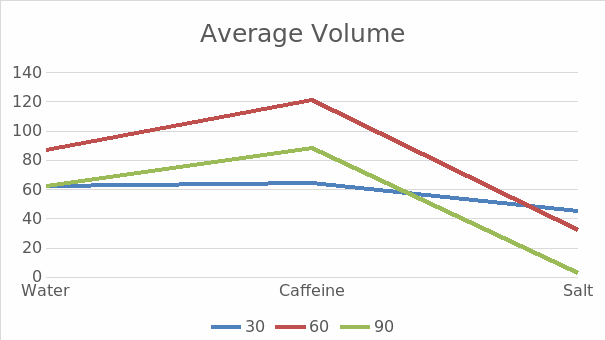
Compared with water, coffee has a diuretic effect. Therefore, it forces the kidneys to work in an enhanced mode, as seen in Figure 1. Drinking coffee does not cause stagnation and requires renal structures to work more actively. Salt is a substance that can detain water and direct it to the kidneys so they can remove it from the body. When the participants of the experiment consumed salt, there was an increase in the volume of urine. Thus, the amount of urine excreted depends on several factors. This is the importance of the liquid received and the concentration of substances that the body must remove, such as salt or caffeine.
Discussion Section
Critical physiological theories are related to the excretory function of the kidneys, which manifests itself in the removal of end products of metabolism and foreign substances from the body. According to the homeostatic theory, it allows for regulating water-salt metabolism and acid-base balance (Li et al., 2018). The different excretion of substances affects the systemic blood pressure and the exercise of endocrine function, manifested in the synthesis of several hormones and the regulation of erythropoiesis (Silva et al., 2021). In connection with the theories considered, the intensity of kidney functioning depends on the characteristics of substances entering the body.
Frequent urination results when drinking coffee are associated with vasoconstrictive properties. The fluids react differently because these coffee properties only enhance filtration in the kidneys’ glomeruli, due to which the fluid is excreted faster than plain water as urine is stimulated (Thummaporn et al., 2020). With moderate use, caffeine does not retain moisture in the body since it belongs to the colloid. Due to its effect, water is involved in the vascular bed, which only increases the excretion of its excess.
When drinking coffee moderately, the body does not suffer from dehydration and fluid balance disorders. Dosed coffee consumption does not interfere with fluid regulation. To prevent the body from dehydrating, it is necessary to drink more water to restore balance. As seen from the experiment, with moderate use of the alkaloid, the frequency of urination increases, not the amount of fluid produced by the body (Belzile et al., 2019). However, excessive consumption of a caffeinated product reduces the amount of liquid. Oversaturation with alkaloids leads to the fact that the accumulated moisture is lost.
Excessive caffeine consumption can negatively affect renal physiology in the body. Stimulating urine production by the kidneys and irritation of the bladder with a large amount of caffeine leads to increased diuresis (Codes et al., 2018). A significant loss of fluid threatens to disrupt the water-salt fluid balance. A constant excess of a tonic alkaloid can provoke urinary incontinence in a healthy person. As the experiment results show, salt retains water in the body: the liquid drunk when using salt is excreted longer than plain water. This is due to the fact that it takes part in reactions with protein compounds. Each sodium ion absorbs four liquid molecules (Wall et al., 2020). Then the water drank back reduces the osmotic pressure of the blood, and the production of vasopressin stops. This mechanism illustrates how salt and edema are related and fluid retention with excessive salt intake.
Normalized salt intake prevented the appearance of excessive water content in the bloodstream and disturbed fluid balance, which would make arterial vessels work faster. This is why it reacts differently than coffee or plain water. This, in turn, could lead to the fact that the vessels would stop narrowing and expanding in time. As a result, a person’s blood pressure increases, and the likelihood of stroke increases. Given the significant effect of sodium on the processes of renal physiology, it is necessary to limit the use of this substance. The adverse effects of excess salt intake are associated with the involvement of the renal system (Thummaporn et al., 2020). Excessive salt violates water-salt, hemodynamic, and endocrine-intracrine homeostasis regulations.
Significant inhibition of glomerular filtration rate control, as the basis of the peritubular factor, is considered the most significant damage to renal physiology. The experiment results were expected and similar to those obtained from previous scientific studies conducted by Li et al. in 2018 or by Belzile et al. in 2019. These studies also revealed a similar dependence of the speed of the excretory system on the liquids consumed. The most important indicators for assessing the urinary function of the kidneys are the volume of primary urine and renal blood flow. Their values for the time ratio and the amount of coffee and salt consumed in this experiment and previous studies were proportional.
Conclusion
The decay products formed in the metabolism process must be removed from the body. This is a necessary condition for vital activity since their accumulation causes self-poisoning of the body and death. The renal system participates in the elimination of substances unnecessary to the body. The experiment proved that depending on the amount of caffeine and salt consumed; they can affect fluid balance and activity of the renal system.
Reference List
Belzile, M., Pouliot, A., Cumyn, A. and Côté, A. M. (2019) ‘Renal physiology and fluid and electrolyte disorders in pregnancy’, Best Practice & Research Clinical Obstetrics & Gynaecology, 57(9), pp. 1–14.
Codes, L., Souza, Y. G., Oliveira, R. A., Bastos, J. L. and Bittencourt, P. L. (2018) ‘Cumulative positive fluid balance is a risk factor for acute kidney injury and requirement for renal replacement therapy after liver transplantation’, World Journal of Transplantation, 8(2), pp. 44–51.
Dalal, R., Bruss, Z. S. and Sehdev, J. S. (2021) ‘Physiology, renal blood flow and filtration’, National Library of Medicine, 34(3), pp. 364–383.
Li, C., Wang, H., Liu, N., Jia, M., Zhang, H., Xi, X. and Hou, H. (2018) ‘Early negative fluid balance is associated with lower mortality after cardiovascular surgery’, Perfusion, 19(1), pp. 1–18.
Masuda, T., Muto, S., Fukuda, K., Watanabe, W., Ohara, K., Koepsell, H., Vallon, V. and Nagata, D. (2020) ‘Osmotic diuresis by SGLT2 inhibition stimulates vasopressininduced water reabsorption to maintain body fluid volume’, Physiological Reports, 8(e14360), pp. 1–12.
Silva, W. A., Pinheiro, A. M., Lima, P. H. and Malbouisson, L. M. (2021) ‘Renal and cardiovascular repercussions in preeclampsia and their impact on fluid management: A literature review’, Brazilian Journal of Anesthesiology, 71(4), pp. 421–428.
Thummaporn, N., Serpa, N. A., Zwakman-Hessels, L., Yanase, F., Eastwood, G., Murugan, R., Kellum, J. A. and Bellomo, R. (2020) ‘Mediators of the impact of hourly net ultrafiltration rate on mortality in critically ill patients receiving continuous renal replacement therapy’, Critical Care Medicine, 48(10), pp. 934–942.
Wall, S. M., Verlander, J. W. and Romero, C. A. (2020) ‘The renal physiology of pendrin-positive intercalated cells’, Physiological Review Journal, 100(23), pp. 1119–1147.