Introduction
Any object that exists in this world is composed of atoms. Timber, water, air, metals and anything else that exists in this world is thus made up of atoms. Atoms are the basic or the fundamental building blocks of all items. Scientifically, all objects existing in this world are referred to as matter.
Therefore, atoms are defined as the “basic building blocks of matter” (Wong 1). From the definition, it is apparent that atoms cannot be divided into smaller particles since they are the basic particles. This property of atoms was the motivation behind their name because the word atom is a Greek word meaning indivisible. At this point, it is important to note that breaking of an atom will result in a change in the chemical composition of the atom. A number of atomic applications use this idea to make use of atoms. This paper explains what atoms are, investigates the history of atoms, looks into the intricate details about the structure of the atom, and it also gives the areas that atomic knowledge has been applied.
History of atoms
The history of atomic study gives an insight of how philosophers’ come up with useful ideal. The model of the atom does not give an absolute atomic understanding, but it gives an abstract methodology that has been used to make useful scientific predictions and explanations (Walker 1). The father of atomic knowledge is a Greek philosopher named Democritus who developed the atom from the idea that breaking of a piece of matter to form two pieces and repeating the same severally must end at a point when the matter cannot break any further. He explained that at this point, one would have the basic particle of matter, which he named the atom. However, his ideas were dismissed by influential philosophers like Aristotle and thus they were not advanced for many years. This happened around 460 years BC. During the 1800’s John Dalton from England showed that matter was indeed made up of lumpy fundamental particles (Fowler 1). J.J. Thompson was the first scholar to come up with a model of the atom after discovering the electron. He discovered that the electron was negatively charged and thus he suggested that the atom has a positive charge. Thompson’s atomic model was conceived in the year 1897. Three years later, another physicist, a German professor named Planck, showed that atomic vibrations produced measurable energy. In the year 1905, a physicist named Albert Einstein showed that absorption of light by atoms is able to release electrons from atoms, by a process known as photoelectric effect. His photoelectric invention earned him a “Nobel Prize for physics in 1921” (Walker 1). In the year 1911, Earnest Rutherford discovered the proton, the particles that make up the nucleus and most of the mass of an atom. In the year 1912, Bohr, a Danish physicist came up with the atomic theory that explains some of the unclear issues surrounding the Rutherford atomic model. One of those issues is why the electrons do not collapse into the nucleus, which Bohr explained by saying that electrons orbit the nucleus of the atom in energy levels.
As you can see, the history of the atom is long. A number of other scholars made great contributions to the idea of the atom. The names mentioned in the above paragraph were the earliest scholars and philosophers who made contributions towards the idea of the atom. Other people who made commendable contributions to the idea of the atom in the later years are Arnold Sommerfeld, Wolfgang Pauli, Louis de Broglie, Erwin Schrodinger, Max Born, Werner Heisenberg, Paul Dirac, Carl Anderson, Hideki Yukawa, Cecil Powell, Richard Feynman, Julian Shwinger, Sin-Itiro, Murray Gell-Mann, Yuval Ne’man (Fowler 1).
Structure of atoms
Atoms are composed of three particles with different properties. These are the protons, the electrons, and the protons. As mentioned earlier, electrons are negatively charged particles that are very light and contribute the least to the atomic mass. Protons are heavy and contribute to a large proportion of atomic mass. Unlike the electrons, the protons have a positive charge. The third type of particles; the neutrons are heavy and contribute a large proportion of atomic weight. As their name suggests, the neutrons have no electric charge and thus they are neutral. Each atom is composed of these particles, which are arranged in a given combination (Gagnon 1). For instance, a hydrogen atom, perhaps the lightest atom, is composed of two particles: one electron and one proton. The structure of the aforementioned atom is thus as shown below:
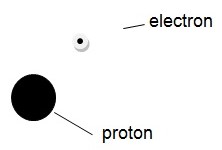
It is important to note that atoms are very small. A hydrogen atom, illustrated above, has an approximate diameter of 5 x 10 -8 millimeters (Carpi 1). Protons and neutrons are similar in most of their properties. They both behave like miniature billiard balls. On the other hand, electrons have the characteristics of waves. The atomic structure can therefore, be depicted as a nucleus, which is positively charged and surrounded by a negatively charged wave. The diagram above would, therefore, be more accurate if the electron is shown as a cloud surrounding the protons. It is also important to note that the atoms of other elements (substances) may have many protons, neutrons and electrons, although the configuration of the particles in the atom is similar for all atoms. With this fact, one may wonder why the nuclei of atoms with multiple protons do not disintegrate. The reason the nucleus does not disintegrate is that every nucleus with more than one proton has an equal number of neutrons that hold the neutrons together (Carpi 1). The latter serve this purpose because the protons are supposed to repel by the laws of electromagnetism. Additionally, electronically neutral atoms have the same number of electrons and protons, which have opposite electromagnetic charges.
Atoms that have a large number of sub-atomic particles (neutrons, electrons, and protons) are larger than their counterparts that have fewer sub-atomic particles. This is the reason why the hydrogen atom was earlier on introduced as the lightest atom.
This discussion has so far highlighted the atomic structure of neutral atoms. It is however, possible to have atoms that have electrical charges. Since the positive charge of an atom cannot be changed, the acquisition of an electrical charge in an atom is only possible through the loss of acquisition of electrons. An atom that has gained an electron will have more electrons than protons and thus it will bear a negative charge. Similarly, an atom that has lost an electron will have more protons than electrons and thus it will have a positive electric charge. An atom that bears a charge is known as an ion (Carpi 1). The following diagrams illustrate two hydrogen ions:

H+: This hydrogen ion bears a positive charge.
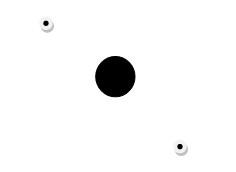
H–: This hydrogen ion bears a negative charge.
Unlike the protons, the number of neutrons in the nucleus of a given atom can be changed. Two atoms that have different numbers of neutrons are known as isotopes. It is thus apparent that isotopes have different atomic masses. A hydrogen isotope known as deuterium is shown below.
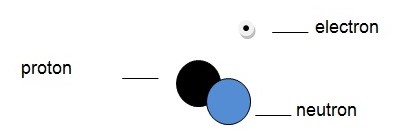
Applications of the atom
Among the main applications of the invention of atoms is the generation of electricity that was possible due to atomic knowledge. Atomic knowledge has also been used by states to get military power through production of atomic bombs. Atom-related inventions like the photoelectric effect have had a variety of applications like their use in image sensors, photomultipliers, and spectroscopy (Freudenrich 1). Other physical properties like electromagnetism have been used in a number of applications and they have their root in the invention of the atom. The invention of the atom made scientists understand the properties of different elements. This has made scientists use different elements in different applications. For instance, radioactive materials, which were better understood because of the prior inventions about the properties of the atoms, are used to generate electricity. Other elements like gold, silver, and copper were better understood and their properties like being shiny and being non-reactive utilized well. Other inventions like the Cathode Ray Tube, which has been used to make various electronics like television sets, computer monitors, etc., have been possible due to the invention of the atom. From this discussion of the applications of the atom, it is apparent that the applications are as many as the various atom-related inventions that followed the invention of the atom.
Conclusion
The atom is among the scientific ideas that have attracted the most scientific research in the 19th and 20th century. Most of the inventions related to the atom were characterized with so much criticism that some of the inventors did not believe in them. For instance, the first inventor of the positron, an electron with a positive charge did not believe that his invention was true. Additionally, after the invention of the atom, a great and influential Greek philosopher – Aristotle – dismissed the invention, making the invention of the atom stay without advancement for centuries. The atom is also among the greatest scientific ideas ever invented in terms of its application. The invention of the atom led to other innumerable inventions that have greatly been applied to make life easier for human beings. The atom is, perhaps, the greatest invention of all times.
From the discussion above, it is apparent that the atom is composed of three sub-atomic particles: the neutrons, the electrons, and the protons. The hydrogen atom is the lightest and the most basic atom consisting of one proton and one electron. This property has made hydrogen to be popular among scholars who use it in their experiments. It is especially used in experiments that investigate atomic properties.
One atomic property that has made the atom to be of great use to scientists and humans as a whole is the ability to change the structure of atoms by creating ions and isotopes. An ion has been defined in this discussion as an atom with a positive charge while an isotope is negatively charged.
Works Cited
Carpi, Anthony. Atomic Structure. 1999. Web.
Fowler, Michael. Evolution of the Atomic Concept and the Beginnings of Modern Chemistry. 2008. Web.
Freudenrich, Craig. How Atoms Work. 2011. Web.
Gagnon, Steve. What are atoms. 2011. Web.
Walker, Jim. Atoms (A short history of the knowledge of the atom). 2004. Web.
Wong, Ling. Structure of the atom. 2010. Web.