The scientific method provides an approach to problem-solving using observations and experimental results. This evidence-based practice allows chemists to draw logical conclusions through examination of data. In this experiment, the scientific method will be applied to determine the factors that affect the rate at which effervescent tablets dissolve in water. Then a hypothesis will be developed and tested subsequently.
Oftentimes, scientists can develop a mathematical correlation between measurements by plotting a graph to represent the data collected. When making such a graph, it is important to recognize the different variables that are used. The independent variable is the factor that is being manipulated so observations can be made and the outcomes of a predicted hypothesis can be measured. The dependent variable is the factor that results from completing the experiment and collecting data which rely on the independent variable. The relationship between the independent variable and the dependent variable can be viewed as a cause and effect relationship. “How does changing A to B (independent variable) affect C (the dependent variable)?” Because it is necessary to only change one variable at a time, all other factors of an experiment must remain the same. These factors are called controlled variables. For example, when studying the behavior of gases to determine the relationship between volume (dependent) and temperature (independent), the amount of gas (controlled) and the pressure of the gas (controlled) must be kept constant.
It will be important to keep in mind what the independent, dependent and controlled variables are in this experiment.
Outcomes
- Construct a hypothesis that can be tested through experimental observations.
- Be able to determine the dependent and independent variables in outlining a lab procedure.
- Apply the steps of the scientific method to find out the validity of a hypothesis through experimentation and analysis of results.
Materials List
From Lab Kit
- Alka-Seltzer tablets, 5 packs
- Beaker, 250 ml
- Wax pencil
- 6 plastic cups
- Thermometer

Other Materials
- Medium-sized glass or bowl for an ice water bath
- Hot water
- Stopwatch (capable of measuring to the nearest 0.1 second)
Safety
Safety goggles should be worn at all times. Do not consume the Alka-Seltzer tablets provided in this experiment. Keep all items away from children and pets. Wash your hands with soap and water before and after performing the lab. Clean the work area and all glassware with soap and water when finished with the experiment.
Temperature and Time
Procedure
- Make an ice bath by placing at least 300 ml of water in a medium-sized bowl and adding about 150-ml of ice. Allow this mixture to stand for 5-10 minutes.
- Obtain a timing device such as a stopwatch or cell phone.
- Label three cups with the wax pencil: RT for room temperature, H for hot, and C for Cold.
- Open the Alka-Seltzer packages. Carefully, with scissors or a knife, scratch a line down the middle of each tablet so it can be broken into halves.*
- Break each tablet into two equal pieces. *By taking a sharp knife or scissors and repeatedly scoring a line across each tablet, it will make it easier to break it in half. The deeper the groove, the more likely it will split into two pieces. If your tablets are already broken, try to create three equal-sized pieces to use for one of the trials. Make a note of this in your data table.*
- Use the 250-ml beaker to measure out 200 ml of room temperature tap water and pour into the “RT” cup.
- Use the 250-ml beaker to measure out 200 ml of cold water into the “C” cup. Do not transfer any ice to the cup.
- Turn your water faucet to hot and let it run for 1-2 minutes ensuring that the water is as hot as it can get. Measure 200 ml of water using the 250 ml beaker and transfer it to the “H” cup.
- Record the temperature of the water in each of the three cups. Include the correct number of significant figures and units in the Data Table 1.
- Carefully place a piece of Alka-Seltzer tablet into each cup, beginning with the “C” cup, then the“RT” cup and lastly, the “H” cup.
- Start the timer immediately.
- Observe the tablets as they dissolve and record what you see. Once the solution has become clear and no more solid pieces are floating around, record the time to the nearest second for that cup in Data Table 1.
- Discard the Alka-Seltzer solution down the drain with running water and rinse the cups multiple times with clean water.
- Repeat steps 6-13 for two more trials keeping the amount of water in each cup at exactly 200 ml. Remember to note if you are using less than a half of a tablet.
- For each temperature range, calculate the average temperature and the average time it took to dissolve the tablet.
Data Table 1
Questions
- Write a statement that represents the hypothesis that was tested in Part A.
The tablet dissolution reaction is quicker in water with higher temperatures.
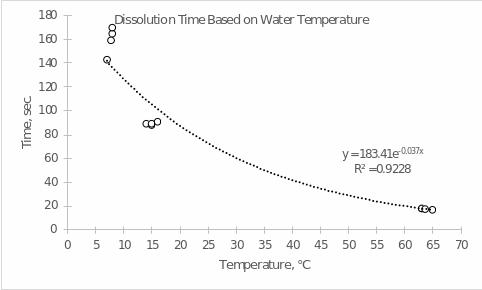
- Prepare a computer-generated graph for the data in Part A. Use all of the values that were tabulated. Include an exponential trendline that shows the mathematical relationship between your measured values. Attach this graph to the lab report. Refer to the Introduction to Graphing Lab procedure for help in creating your graph.
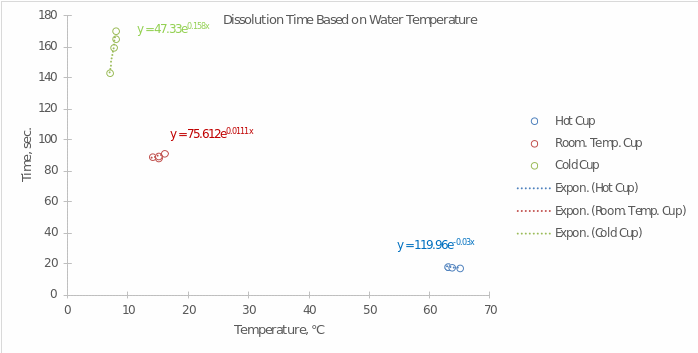
What is the independent variable? Explain why you chose this and which axis it should be on.
In this experiment, the independent variable is the water’s temperature. It should be placed on the x-axis since it is through temperature manipulation, we are able to influence the speed of the reaction.
What is the dependent variable? Explain why you chose this and which axis it should be on.
Dissolution time was chosen as the dependent variable since this factor was the key interest for the experiment. Manipulating the temperature was to affect the dissolution time, according to the hypothesis.
What are the controlled variables? Provide at least two.
The controlled variables of this experiment are the Alka-Seltzer’s composition, water amount, and the amount of Alka-Seltzer used in each trial – 1 tablet.
- Using the equation generated in your graph, determine the amount of time it will take for the tablet to dissolve at the following temperatures.
- 16 ℃ 183.41×10-0.037×16 = 46.93 sec.
- 42 ℃ 183.41×10-0.037×42 = 5.12 sec.
- 87 ℃ 183.41×10-0.037×87 = 0.11 sec.
Testing a New Hypothesis
Questions
- List 4 questions about the speed in which the Alka-Seltzer tablets dissolved. (Think about interchanging a controlled variable with an independent or dependent variable.)
- Question #1 – How will the dissolution time change if the water is replaced with another solution?
- Question #2 – How will the dissolution time change if the tablet amount changes?
- Question #3 – Is the dissolution rate the same for different types of dissolvable tablets, given the equation constructed?
- Question #4 – Is there a limit at which the temperature no longer influences the dissolution time?
- Create a new hypothesis based on one of the questions from above and fill out the following:
- Stated hypothesis – Alka-Seltzer will dissolve quicker in a hydrochloric acid solution than in water, regardless of temperature changes.
- Independent variables – Dissolving solution, temperature
- Dependent variable – Dissolution time
- Controlled variables – Alka-Seltzer composition and amount for each trial
- How will the hypothesis be tested? Experimentally. For this, several cups with the same volume of two solvents are used, they are heated to different temperatures (e.g., 5°C, 25°C, and 60°C), then the time required to dissolve the same number of tablets in each of the solvents is measured. Based on the results, the hypothesis is confirmed or rejected.
- What are the expected results? The Alka-Seltzer dissolution will be quicker in hydrochloric acid at any temperature than in water.
Procedure
Write a procedure to test your new hypothesis. Include step-by-step instructions. Provide a list of materials. Be very specific in how to run the experiment so that another student could perform it as written. Make sure to include a description on the number of trials so the results show reproducibility.
Materials List
- Alka-Seltzer tablets, 6 packs
- 18 beakers, 250 ml
- 1 bottle of water (2 L)
- Ice cubes
- Hydrochloric acid (2 L)
- Hot plate
- Stopwatch
- Thermometer
Fill three beakers with 200 ml of water and use ice to lower the temperature to 5℃. Place one Alka-Seltzer tablet in the water, measure the time it takes for the tablet to dissolve completely using a stopwatch, and record the results in the table.
Fill three beakers with 200 ml of water and heat them with a hot stove to 25 ℃, respectively. Place one Alka-Seltzer tablet in each beaker, measure the time it takes for the tablet to dissolve completely using a stopwatch and record the results in the table.
Fill three beakers with 200 mL of water and heat them with a hot stove to 60 ℃, respectively. Place one Alka-Seltzer tablet in each beaker, measure the time it takes for the tablet to dissolve completely using a stopwatch, and record the results in the table.
Repeat steps 1-3 but use hydrochloric acid instead of water.
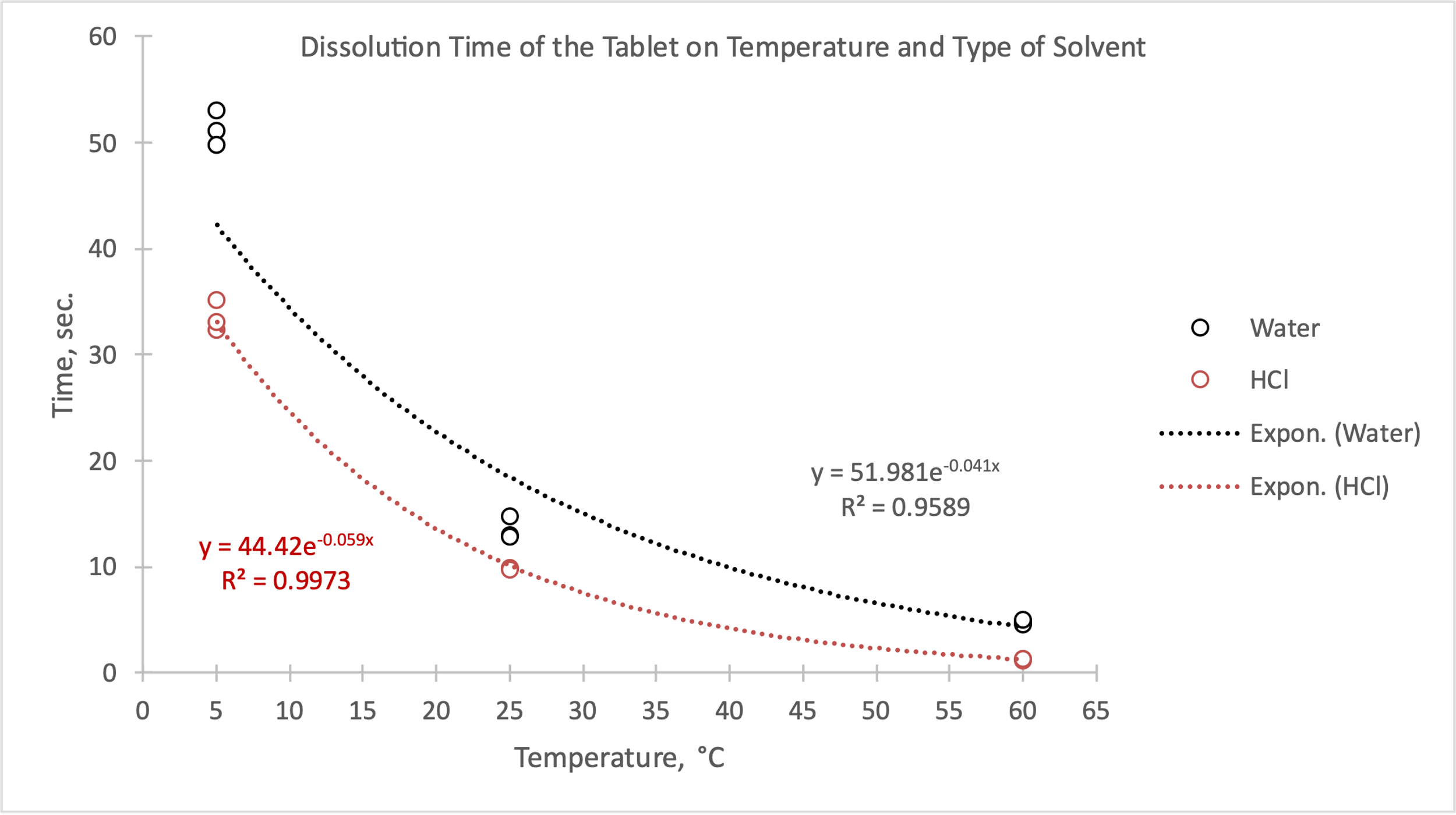
Following the outline of your procedure, run the experiment and record the results in a table of your design in the space below. It should be similar to the one in Part A. Label it Data Table 2.
Data Table 2
Cleaning and Waste Disposal
- All solid waste should be thrown away into a garbage can.
- All liquid waste should be washed down the drain with running water.
- Wash the cups, beaker, and thermometer. Wipe down the workspace. Wash your hands.
Post-Lab Questions
- Discuss the results of your experiment in Part B. Was your hypothesis correct? Are there changes that should be made to your procedure? Explain.
The results of the second experiment show that the proposed hypothesis is correct. The dissolution of the tablet in hydrochloric acid is indeed faster, as evidenced by the constructed regression equation. A potential reason for this is the sodium carbonate contained in Alka-Seltzer, which starts the reaction with the acid, which, compared to the acid, makes the reaction go more intensely. Although the experiment showed success, a possible change that could be considered is to increase the number of repetitions to reduce sampling error or bias.
- What are two things that could be done to improve the reproducibility of your results in Part A and Part B?
It is necessary to ensure that the set temperature is maintained at all times in order to reduce error.