Objective of the experiment
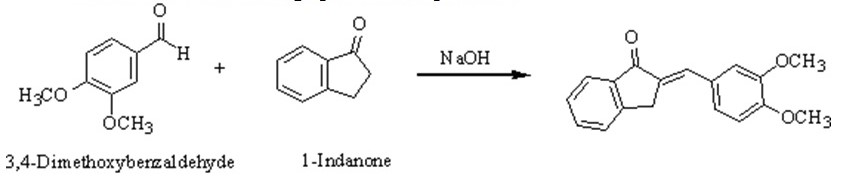
To explore the base-catalyzed aldol condensation between 3, 4-dimethoxybenzaldehyde, and 1-indanone under solvent-free conditions;
To purify the product through recrystallization;
To evaluate the melting –point of the product.
Procedure
The reaction procedure is carried out by mixing a mass of 0.25g of 3,4-dimethoxybenzaldehyde (white solid), with 0.20 g of i-indanone (green color) in a test tube. Using a metal spatula, the two solids were crushed together until brown-colored oil was formed. Then, 0.05 g of finely ground, white solid crystals of NaOH were added to the reaction mixture. The mixture was constantly mixed with the spatula until it became solid and finally, the mixture was allowed to stand for 15 minutes at room temperature. This caused it to cool down. To purify the solid product, 2 mL of 10% aqueous HCl solution was added, and the product isolated through vacuum filtration and dried. The mass of the crude product is measured and its melting point determined. The product was recrystallized from a mixed solvent of 90% ethanol and 10% water. The mass and the melting point of the recrystallized product were then determined.
Data
- Weight of aldehyde used: 0.25 g.
- Weight of ketone used : 0.2 g.
- Volume of NaOH used : 0.05 g.
- Volume of 10% HCL used: 2 ml.
- Volume of 90:10 ethanol/water used for recrystallization: 20 ml.
- Final product weight : 0.22 g.
- Experimental melting point: 178.
- Atom Economy: (Mass of Product/ Sum of Mass of Reactants) * 100 = 94%.
Discussion of the experiment
A typical aldol condensation reaction is carried out in an organic solvent, such as ethanol, requiring eventual waste disposal. One of the main themes of greener chemistry is to cut down on the use of solvent and hence cut down on solvent waste. The best way to achieve this is by avoiding the use of any solvent. A unique feature of this particular reaction is that it does not make use of any solvent between two solids. Hence this reaction is very benign and it is very atom economical. Moreover, the separation of the product is easy as the reaction proceeds as the product separates from the melt as a solid. Another benefit is that, unlike a solution-phase reaction, the solid-state reaction is irreversible, resulting in higher chemical yields. The Aldol condensation if affected without dehydration has an atom economy of 100%, requires only a catalytic amount of acid or base, and even with dehydration, the atom economy is high. This purification method takes advantage of the differences in solubility between the compound and its impurities. Most of the impurities will remain dissolved in the cool solvent, allowing them to be removed when the sample is isolated by vacuum filtration. Some of the impurities may not dissolve even in hot solvent, requiring a hot filtration to remove them.
Results
After filtration through a vacuum, a mass of 0.22 g of crude product was obtained of yellowish to green color.