Introduction
The purpose of this experiment is to determine the pH of the solution at the endpoint as well as finding the concentration of each of the acids using a primary standard solution of known concentration. This basically means determining the exact amount of acid, which is chemically equivalent to the amount of base present. The point at which chemically equivalent amounts of acid and base are present is known as either the equivalent point or the endpoint (Laubengayr, 1999).
The pH of a solution at the equivalence point depends upon the nature of the acid and the base being titrated. If both are strong electrolytes, the solution will be neutral (i.e. [H+] = [OH–] and will have a pH of 7. If a weak acid is titrated a strong base, there will be the hydrolysis of the anion of the acid. The solution will be basic; this means the pH will be greater than 7. Similarly, for titration of a strong acid with a weak base, hydrolysis occurs, and the solution will have a pH less than 7 (Pikens, 2000).
A– + H2O = HA + OH–
M+ + H2 = MOH + H+
The most important method of detecting the endpoint of an acid-base titration involves the use of pH indicators. These are substances, weak organic acids, or bases, which possess different colors according to the pH of the solution.
Method/procedure
Open the data window and click on the chemical shelf to select 0.1M sodium hydroxide (NaOH) and HCl of an unknown concentration (Late Nite Labs, 2011).
Titration of strong base with strong acid I
Place a clean Erlenmeyer flask on the workbench. Open the Chemical Shelf and add 25 mL HCl (of unknown concentration) to the flask. Add 2 drops of phenolphthalein to the flask. Place a clean burette on the workbench. Fill the burette with 50 mL of 1M NaOH solution. Click on the flask from the data window. Lock the display by clicking on the pushpin button. Insert a pH meter from the tool section into the flask.
Read and record the initial value of pH. Click on the Properties window of the burette and enter “1” in the amount to add, and click the flow button to drip 1 mL of NaOH into the flask. Record the pH of the solution in the flask. Continue adding sodium hydroxide in increments of 1 mL. Record the pH for each milliliter added. The pink color will appear in the flask all at once when the endpoint is either reached or crossed. Record the burette volume and pH at which this occurs. Continue to add 5 more increments of 1 mL and record the pH at each point. Detach the flask from the burette and drag it to the recycling chute.
Take a new flask from the Labware Shelf, under the Glassware Tab, and place it on the workbench. Add 25 mL of HCl and 2 drops of phenolphthalein to the flask. Refill the burette to 50 mL NaOH. Based on the results of the previous titration, add enough NaOH solution – all at once – to get to 1 mL BEFORE the endpoint. Using the NaOH from the Chemical Shelf, add.0001 mL to the solution until you reach the endpoint. Record the pH and volume until the endpoint is reached and several drops after it are reached.
Titration of acetic acid with Sodium Hydroxide
Detach the flask from the burette and drag it to the recycling chute. Place a clean Erlenmeyer flask on the workbench. Add 5 mL of acetic acid (of unknown concentration) to the flask. Add 20 mL water and 2 drops of phenolphthalein to the flask. Refill the burette with the NaOH solution, and record the initial volume. Drag the flask and drop it on the lower half of the burette to connect them. Insert a pH meter from the tool section into the flask.
Record the initial pH of the solution. Add NaOH in 1 mL increments and record the pH for each milliliter added. The color will change at once when the endpoint is passed or reached. Note the burette volume and pH at which this occurs. Continue to add 5 more 1 mL increments of NaOH into the flask. Record the pH after each increment. Detach the flask from the burette and drag it to the recycling chute. Place a clean flask on the workbench.
Add 5 mL of acetic acid, 20 mL water, and 2 drops of phenolphthalein to the flask. Refill the burette to 50 mL NaOH. Drag the flask and drop it on the lower half of the burette to connect them. Drop the pH meter into the flask. Depending on the results of the coarse titration, add a sufficient amount of the base just before the volume for the endpoint. Enter 1 as the amount to add and add NaOH DROP-WISE into the flask. Record the pH and volume until the endpoint is reached and several drops after it are reached.
Results
Equivalent point.
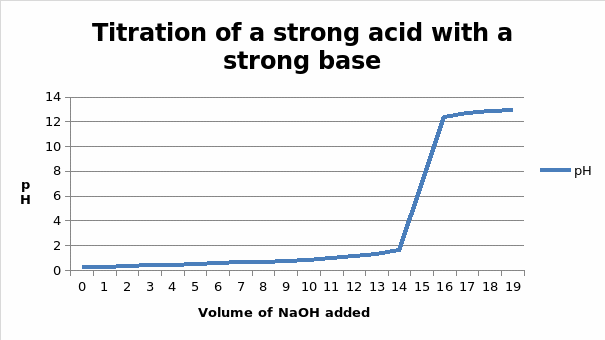
The end point of the coarse titration is 16 ml, while the end point of the fine titration is 15.0005mL
At the half equivalent point, the moles of the acid that are present in the solution are half the total moles present at the equivalent point. Therefore the moles present at this point will be 0.00150005/2 = 0.0007525 moles
The volume of NaOH that corresponds to this number of moles is going to be (moles/molarity) * 1000 = (0.0007525/ 0.1) * 1000 = 7.50025ml
From the graph, this volume gives a pH of 0.63
At the equivalent point, the volume of the base added is 15.0005ml. The number of moles in this volume will be MV/1000
- (15.0005 * 0.1)/ 1000 = 0.00150005 moles
Since the mole ratio of the acid and the base is 1:1, then the acid will have an equal number of moles as the base. Therefore to find the molarity of the acid at the end point, the moles will be divided by the volume. The volume of the acid is 25ml
M1V1 = M2V2 Where 1 represents HCl and 2 represents NaOH
Therefore M1 = (15.0005 × 0.1) ÷ 25 = 0.06002 M
Weak acids and bases do not completely ionize compared to strong acids and bases.
- Ka = [H+] [Cl-]/ [HCl] [H2O] is the dissociation constant for a strong acid, HCl. But since it dissociates completely, HCl does not exist in the solution. Therefore the equilibrium shifts to the right
- Ka = [H+] [OAc–]/ [HOAc] [H2O] is the dissociation constant for a weak acid, Acetic acid. Since it fails to dissociate completely, the concentration of the acid in the solution could still be identified. The equilibrium of the solution shifts to the left.
Acid base titrations need to be followed precisely according to the instructions provided in order to avoid mistakes and contaminations. Contaminations can cause the experiment to be flawed. Titration as a volumetric technique of chemical analysis is used to determine the concentration of a solution that has unknown concentration using a standard solution. This can only achieved accurately if the procedure is followed precisely.
References
Late Nite Labs. (2011). Laboratory simulation for science education. Web.
Laubengayr, W. (1999). Experiments and problems in general chamistry. Boston: McGraw Hill.
Pikens, D. (2000). Intergrated experimental chemistry. New York: Pearson.