X-ray fluorescence (XRF) is an investigative method that ascertains the chemical composition of a substance by identifying the individual elements present. However, the sample remains intact during the entire analytical process (Tykot 2016). A special type of equipment referred to as an XRF analyzer verifies a sample’s chemical makeup by measuring the level of fluorescence given off when the material is excited by X-ray radiations (Goldstein et al. 2017). XRF is a flexible method that can be used successfully on solid or liquid samples. Furthermore, simple sample preparation steps are needed before analysis, which has made XRF the preferred analytical tool in qualitative and quantitative investigations involving geological materials (Sharma et al. 2015; Lutterotti et al. 2016).
An X-ray tube in the analyzer produces radiations that are absorbed by atoms in the sample. Consequently, the atoms ionize and release electrons from the lower K and L energy levels in a process that destabilizes the atom. The ousted electrons are substituted by electrons from a higher energy level in a process that releases energy in the form of fluorescent X-rays because these electrons have more energy than the previously expelled ones (Anand et al. 2018). The emitted rays are characteristic of each element and can be used to identify them.
Experimental Procedure
About 20 unknown salt samples with unique identification numbers were provided to the class. One unknown sample (number 3) was chosen and used for the subsequent investigations. Two other students also handled the same salt sample. An XRF film was put over a cup, which was covered by a different cup thereby pushing down the film. A clean weighing balance was used to weigh about 2.57 grams of the unknown salt.
It was important to ensure that the weighting balance was clean to avoid introducing any extra material that could interfere with the accuracy of the measurements. The correct weight was expected to range from 2 to 3 grams. The salt sample was then put in a plunger to be compressed followed by measuring the height of the compacted sample, which was 7 cm. Appropriate labeling of the sample was done on the cover of the cup by including pertinent details such as the sample number, student’s initials, weight, laboratory session, and height after compression. The prepared sample was then placed in the RIGAKU XRF machine for evaluation alongside the unknown samples prepared by other students. The results of the analyses were later provided to facilitate the compilation of the report.
Result
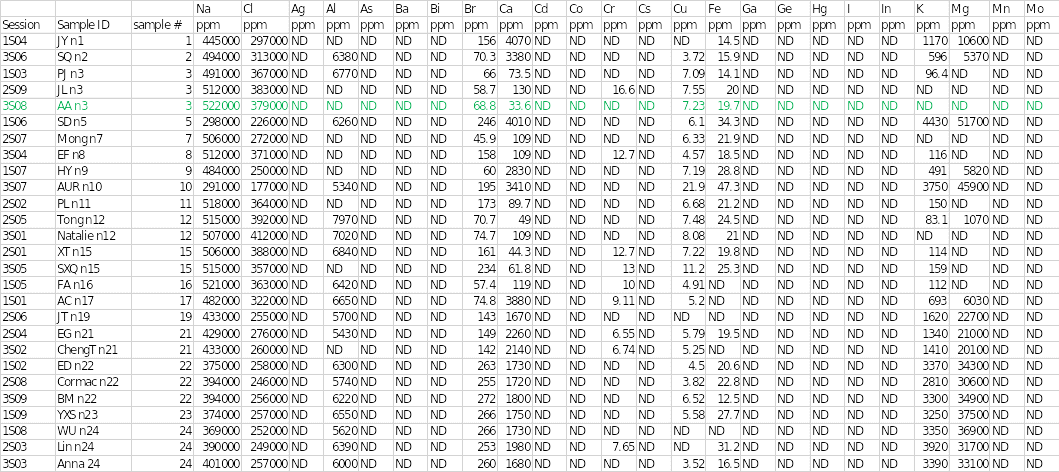
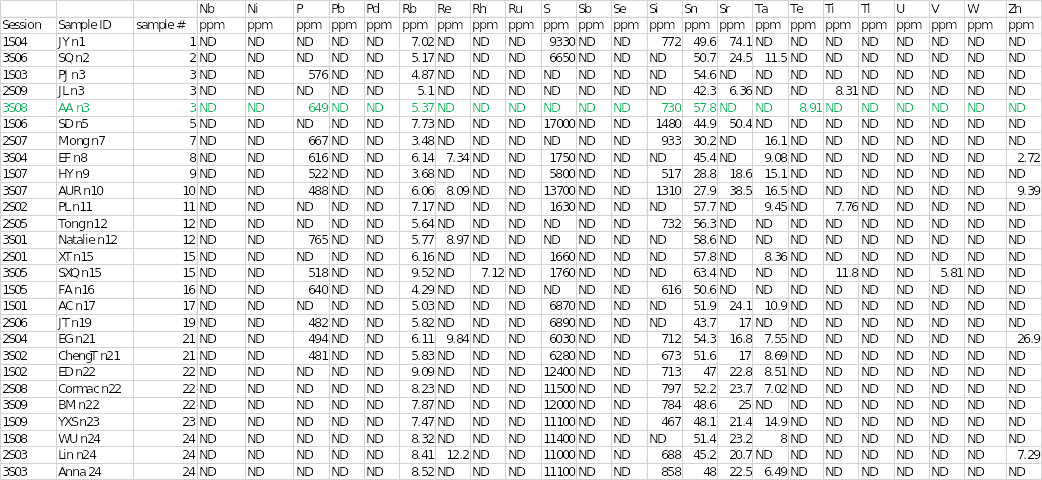
Table 1 below shows the concentrations of different trace elements in the salt samples. The findings of the individual sample that was assigned are highlighted in green. The results showed that sodium and chloride were the main cation and anion at concentrations of 522,000 and 379,000 parts per million (ppm), respectively. Sample 3 had the highest concentration of sodium of all the salts. Mineral elements that were present in sample 3 included bromine at 68.8 ppm, calcium at 33.6 ppm, copper at 7.23 ppm, iron at 19.7 ppm, and phosphorous at 649 ppm.
Other chemical elements that were found in the salt specimen included rubidium, silicon, stannum (tin), and tellurium at concentrations of 5.37, 730, 57.8, and 8.91, respectively. Trace elements that were seen in other samples but were absent in sample 3 included aluminum, chromium, potassium, magnesium, rhenium, rhodium, sulfur, strontium, tantalum, titanium, vanadium, and zinc. In contrast, some elements were not completely detected in any of the salt samples.
They included silver, arsenic, barium, bismuth, cobalt, cesium, gallium, germanium, mercury, iodine, indium, manganese, molybdenum, niobium, nickel, lead, palladium, ruthenium, stibium (antimony), selenium, thallium, uranium, and tungsten. Disparities existed in some of the elements identified in the other two duplicate analyses. For example, aluminum and potassium were detected in session 1S03, whereas chromium, strontium, and titanium were observed in session 3S08.
Discussion and Conclusion
The XRF findings and physical appearance of the unknown specimen indicate that it is likely to be table salt. The industrial processing of table salt involves high levels of refinement to get rid of impurities and grinding into a fine powder (Warren 2016). The pounding increases the tendency of the salt particles to clump together, which was observed in sample 3. Conversely, the refining process removes some of the impurities that contribute to discoloration in salt, thereby resulting in a pure white product (Hopkins 2015). The specimen also contained large quantities of sodium and chlorine, which is usual for table salt (Fang et al. 2019).
The sample could not be sea salt because it lacked sulfur, which makes up sulfates that are found in sea salt. Trace minerals that can be found in sea salt include potassium, bromine, phosphorus, boron, iron, zinc, copper, manganese, and silicon (Yang et al. 2015). Nonetheless, some of these components such as potassium, zinc, and manganese that are commonly found in sea salt were absent in the analyte.
The possibility of the unknown sample being rock salt was ruled out because barium and strontium, the two major elements that are found in rock salt, were absent (Chen et al. 2019). The outcomes of this experiment prove that XRF is a dependable technique in the chemical analysis of the elemental organization of substances.
Reference List
Anand, LFM, Gudennavar, SB, Bubbly, SG & Kerur, BR 2018, K-shell X-ray fluorescence parameters of a few low Z elements’, Journal of Experimental and Theoretical Physics, vol. 126, no. 1, pp. 1-7.
Chen, A, Yang, S, Xu, S, Ogg, J, Chen, H, Zhong, Y, Zhang, C & Li, F 2019, ‘Sedimentary model of marine evaporites and implications for potash deposits exploration in China’, Carbonates and Evaporites, vol. 34, no. 1, pp. 83-99.
Fang, D, Huang, W, Antkiewicz, DS, Wang, Y, Khuzestani, RB, Zhang, Y, Shang, J, Shafer, MM, He, L, Schauer, JJ & Zhang, Y 2019, ‘Chemical composition and health risk indices associated with size-resolved particulate matter in Pearl River Delta (PRD) region, China’, Environmental Science and Pollution Research, pp. 1-11.
Goldstein, JI, Newbury, DE, Michael, JR, Ritchie, NW, Scott, JHJ & Joy, DC 2017, Scanning electron microscopy and X-ray microanalysis, Springer, New York, NY.
Hopkins, J 2015, Everyday nutritional mistakes making you fat: things you need to know about food, that keeps sabotaging your waistline, BookBaby, Pennsauken, NJ.
Lutterotti, L, Dell’Amore, F, Angelucci, DE, Carrer, F & Gialanella, S 2016, ‘Combined X-ray diffraction and fluorescence analysis in the cultural heritage field’, Microchemical Journal, vol. 126, pp. 423-430.
Sharma, A, Weindorf, DC, Wang, D & Chakraborty, S 2015, ‘Characterizing soils via portable X-ray fluorescence spectrometer: 4. cation exchange capacity (CEC)’, Geoderma, vol. 239-240, pp. 130-134.
Tykot, RH 2016, ‘Using nondestructive portable X-ray fluorescence spectrometers on stone, ceramics, metals, and other materials in museums: advantages and limitations’, Applied Spectroscopy, vol. 70, no.1, pp. 42-56.
Warren, JK 2016, Evaporites: a geological compendium, Springer, New York, NY.
Yang, D, Shi, H, Li, L, Li, J, Jabeen, K & Kolandhasamy, P 2015, ‘Microplastic pollution in table salts from China’, Environmental Science & Technology, vol. 49, no. 22, pp. 13622-13627.