Introduction
Plastics are found everywhere and have many uses. However, there are some limitations to the use of plastics and one of these is that they cannot withstand a temperature way above 200°C. Plastics that can withstand high temperature are applied with so-called aromatic base.
This kind of plastic is exposed to condensation during manufacturing and also possesses high melting point. Polyethylene terephthalate is a kind of polymer that has a melting point of 145°C. Others are within the range of 200°C but not higher than that.
Plastics are also limited by the chemical composition they are made. Properties of plastics become limited because of the chemical composition they possess and as such, their hardness and density and other remarkable properties are totally affected.
A lot of plastics commonly used today are made of polymers (or polymer based). Manufacturers utilize various techniques in polymerization and one of these is the application of several additives to vary the plastic properties and to attain the desired results for commercial purposes.
The most likely plastic used
The most likely plastic used today is a combination of several chemicals but one process applied is polymerization. Poly means a combination of several chemicals to produce a type of plastic that has many applications. An example is that some plastics have chemicals with a so-called backbone to introduce quality and to make it more commercially viable and cost-effective.
There are five types of plastics that are commonly used and are described in this paper. These are the polyethylene, polypropylene, polystyrene, vinyl and polyethylene terephthalate. The discussion will focus on these types of plastic.
Polyethylene Plastic (PE)
This is one of the most widely-used plastic because of its valuable properties and many applications.

Figure above shows examples of products made from PE plastic
The origin of polyethylene comes all the way from England before the outbreak of the Second World War. ICI Corporation made the move without the intention to introduce it commercially. They did it through an application of high pressure of ethylene mixed with small amount of oxygen.
Interestingly, an accident occurred at the plant conducting the experiment, but the application of oxygen to ethylene produced accidental but positive results. Polyethylene was born. (Green and Wittcoff, 2003, p. 24)
The first type of polyethylene and also the first to be introduced commercially is the low-density polyethylene (LDPE). It can function on ordinary temperature and whose qualities include strength and flexibility. It can withstand extreme weather condition and does not easily absorb water.
This type of plastic is applicable to situations where corrosion resistance is a factor. The many properties of LDPE make it a valuable commodity in the plastic family. It is more superior to other types when it comes to density. It has the stiffness qualities but also has a soft surface. (Vasile and Pascu, 2005, p. 15)
Another type of polyethylene is the high-density PE resin or HDPE which is applicable for tough and rigid injection mouldings and film products. Other applications for this type of PE are for household and personal care, but also including industrial container and home products. HDPE resin is a valuable chemical, can withstand extreme weather and also has superior qualities important to plastics.
High-density polyethylene has high compression properties and is stronger than LDPE. This type of PE is applicable for food wrappings and can be used for food contact. FDA approved, LDPE has been promoted by other US government agencies like the Department of Agriculture, and the Agriculture Department of Canada. (Vasile and Pascu, 2005, p. 16)
Why was this selected?
Polyethylene (PE) plastic has a particular density of 0.941 g/cm-3 and is popularly used in industries throughout the world. Polymerization is introduced to create semi-crystalline particles which seem transparent. The monomer also helps in the condensation process.
PE’s various applications make it the most in demand. It is the choice of industries and users, because it can be used as substitute for other materials like glass and metal. It can also be used as substitute for paper. Polyethylene products include plastic ropes and fibers.
It is tough, easy to process, and has electrical properties. It has a distinct chemical resistance and does not absorb moisture. It has many applications, like water pipes, petroleum tanks, and other various home materials and utensils. (Vasile and Pascu, 2005, p. 4)
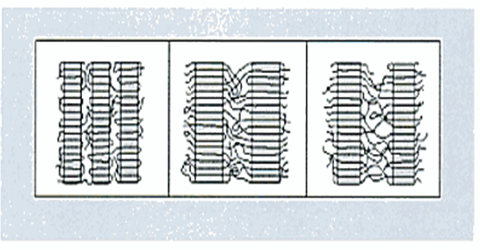
Figure above shows a schematic representation of PE molecules and the different types of PE.
SOURCE: Condensed from “Practical guide to polyethylene”, by Vasile and Pascu (2005, p. 75)
Polypropylene Plastic (PP)
Polypropylene plastic (PP) also uses polymer to produce the qualities of plastic. Polymerization is introduced to produce crystalline molecules. PP is used to create different forms and sizes in car bumpers and other parts. This is particularly very useful for the automotive industry.
Other varied applications of PP include pipes, packaging, and various types of fibers. The chemical term for polypropylene is C3H6 and its melting point can reach up to 160˚C. This is a low-cost plastic and is resistant to high temperature when not applied with great pressure.
Its resistance to other chemicals is quite remarkable and the hardness is useful to some industrial applications. PP is not dangerous to people’s health, unlike some plastics, and can be used to replace polystyrene for various uses. (Tripathi, 2002, p. 3)

Figure above shows an example of polypropylene plastic that can be used as ropes.
Polystyrene
Polystyrene was discovered by the German chemist Eduard Simon when he processed liquid storax which are found in certain varieties of trees. He was the first to use the word styrol for this discovery. The process Simon used was pyrolysis.
The chemical is now popularly known as styrene and has the property of converting itself into solid. Solid styrene was termed by scientists as “meta-styrene”. The process of transformation into solid was clearly explained by Herman Staudinger who said that this was the result of the formation of chain-like molecules called macromolecules. This process is later known as polystyrene. (Wunsch, 2000, p. 7)

The figure above shows several examples of polystyrene. It can be used as paper or styro to contain food and other items needed at home, schools and offices.
In the petrochemical industry today, styrene is processed and produced using the catalyst ethylbenzene and hydrogenation. Ethylbenzene can be processed in the laboratory through “catalytic alkylation of benzene”. This is also used in commercial processing.
Vinyl
Common raw materials to form vinyl chloride are chlorine and ethylene. Chlorine is a product of electrolysis while ethylene can be processed from naptha which is a product of oil or natural gas. Polyvinyl chloride (PVC) is a product of vinyl chloride.
PVC has a low melting point so that manufacturers add some chemicals to polymer to make it stronger. It has now a wider variety of applications and is second to polyethylene when it comes to cost effectiveness. The polymer is added with other chemicals to provide some properties for a variety of applications.

Figure shows several applications for polyvinyl chloride such as various forms of pipes.
PVC chemicals can be processed with the use of raw materials called plasticisers and flexible materials. A chemical known as PVC-P which is in liquid form is added to form the PVC. Metal chlorides are used in the catalytic processing.
Vinyl chloride monomer was discovered in 1835 but the real importance of the chemical was made known in the early 1900s by Fritz Klatte who motivated others to produce and commercialize PVC. Demonstrated to end users was the melting point of PVC at 180˚C.
PVC resins were first manufactured in Germany in the years before the Second World War. Many applications have been discovered from PVC and one of these is that it resembles like rubber or leather when heated to its boiling point. Manufacturers used plasticisers to make PVC more commercially applicable. (Patrick, 2005, p. 2)
Polyethylene Terephthalate (PETP)
There are various types of polyethylene terepthalate. The amorphous type is known as APET while the crystalline form is known as CPET. PETP is used commercially, particularly in packaging and as containers of drinks. In making these containers, a specialized kind of machine is used. PETP’s melting point is high at 245˚C.

One of the many applications of polyethylene terephthalate (PE) is the sailcloth.
What is the most likely process?
In answering this question, it is fit and proper to enumerate the various types of processes used in polymerization and plastic manufacturing.
The Polyethylene Process
Polyethylene is produced through a process called “free radical polymerization” which involves several chain reactions. Large quantities of polyethylene are manufactured and needed in large industries.
Polymeric fibers are made through a process called drawing which is done by allowing the polymer molecules to become like tiny crystals and letting the molecules attract and align with each other. The process also includes diluting hydrocarbon solution of polyethylene at a temperature of 120°C and subsequently cooled to produce gel-like fibers.
After the cooling process, the solvent is separated and another chemical known as dichloromethane is applied. This produces a new fiber known as Dyneema. It is believed that the tensile strength of this new product is a lot greater than steel and the fiber known as Aramid.
Dyneema is a trade name and is available commercially under the name Spectra. Polyethylene fibers have low melting point than the Aramid. They are also slow in absorbing water. (Wittcoff et al., 2004, p. 109)
The Polypropylene Process
On the other hand, polypropylene plastic is processed this way. A catalyst used to make a homopolymer plastic in PP is known as Ziegler-Natta.
In making the right grade for PP in several applications, one has to choose the type of polymer whether homopolymer and copolymer, the kind of reactor and the required melt flow, including other additives required for an application. Manufacturers however have different techniques in selecting the right grade for an application.
Homopolymer is a type of polypropylene plastic which is processed with the use of a different kind of catalyst. It is stronger than copolymer when it comes to heat resistance. Copolymer, on the other hand, is another type of PP whose properties depend on the kind of comonomer added to the raw material when processed.
The Polystyrene Process
The third type is polystyrene. It is processed with the use of a big amount of styrene (about 50%) and other ingredients such as elastomers, resins and polymer. The big demand of styrene worldwide comes from North America, Europe and the countries from East Asia.
Polystyrene is one of the biggest polymer productions of the five plastic types mentioned in this paper. In 1996, Western Europe had the biggest production of styrene followed by Eastern Europe and North America. (Wunsch, 2000, p. 5)
In the processing of PP, a polymer chain is formed. This kind of molecular chain has several characteristics. A polymer material has a particular weight, chemical composition and “stereoregularity” of a polymer behavior.
Vinyl Process
A remarkable characteristic of vinyl is its being a thermoplastic, or the property of a material to soften when heated and harden when the temperature is lowered. The chemical composition however does not change when this process is applied to the PVC. Vinyl is used to make wires and cables, several applications in different shapes and forms, floor covers and decorations, coatings, and many others.
The plasticizer used in vinyl is dialkyl phthalate which has a molecular weight range of 362 to 446. This is for general purposes. In plasticizing vinyl, manufacturers and researchers explain the process through an application of the theories on polymer plasticization.
One theory is the lubrication theory which states that the molecules of a plasticizer are inserted between the polymer molecules. This allows a softer molecular base leading to a softer matrix.
Another theory is the gel theory which states that the polymer molecules is weakened by the plascitizer. The free volume theory states that the softness and flexibility are the results of the plasticizing molecules. (Zweifel et al., 2009, p. 487)
Polyethylene Terephthalate Process
PETP is processed using polycarbonate. The process known as moulding is done in an oversize bottle. A temperature of 220˚C to 230˚C is applied to produce crystalline materials making the bottle shrink until it reaches its desired size. (Barnetson, 1996, p. 7)
The most likely process being asked in this paper could be taken as hypothesis from the discussion above. All of the above processes include a process called polymerization and the adding of a catalyst to produce the transformation process.
In polyethylene, it is called radical polymerization. In producing polymeric fibers, a process is done called drawing and diluting hydrocarbon solution of polyethylene. A new fiber is produced called Dyneema.
In processing polypropylene, another catalyst is added called Ziegler-Natta, while in polystyrene processing, they use styrene added to polymer. Styrene is in big demand in many parts of the world. Vinyl and PETP also use catalysts.
So in answering the question as to what is the most likely process, it is the polymerization with the use of a catalyst to produce quality plastic with many applications.
What are some alternative to plastics?
Plastics are very common and have many applications. But they are non biodegradable materials; meaning they do not change their chemical properties for a very long period of time. Plastics can be replaced with wood or cement. For example, instead of PVC, cement can be used as pipes. Where there are times that plastics replace paper, paper can also replace plastics for the purpose of preserving the environment.
Wood and metal can be used to in window frames instead of the superior strength of PVC. Wood has many applications. It is biodegradable and a quality raw material that is often used at homes and offices. Wood can replace plastic, although if we look at history, wood came first than plastic.
Moreover, there are plastics that are not applied with chlorine. These plastics are stronger than PVC. They are not dangerous during manufacture and final use. PVC was exclusively used for many applications including those commonly used in daily living activities such as packaging of shoes, bags, dresses and a lot more, but polymers which chlorine have been made as substitute. (Thornton, 2000, p. 387)
What are some alternative processes?
Incineration of plastic to acquire new plastic chemicals and re-process them is one of the alternative methods. This process can provide the necessary raw materials but products from recycled materials are of lesser quality than products produced from so-called virgin polymers.
Another thing is that incineration affects the environment because of the carbon dioxide that the incinerator emits. So this alternative process is not a good recommendation.
The process of making liquid wood is an alternative process for replacing plastic. Although still in its infancy, liquid wood can go a long way for the various uses of plastic. It would not be too difficult process because scientists have made the breakthrough. What they need now is polishing this invention to make our environment safer from the use of plastic.
What other material other than plastic could have been used?
Some alternatives have been introduced on the packaging of foods and drinks instead of the usual plastic. However, this depends on the nature of food to be packaged. Naphtha, which is a by-product of natural gas, is a raw material for making olefin. But a fraction of naphtha can also be used to make propylene. Propylene is quite difficult to extract as a raw material. (Wittcoff et al., 2004, p. 58)
We have discussed above of the availability of wood as a replacement of plastic. There is another discovery known as liquid wood that can be used instead of plastic. It is known as arboform from a material known as lignin which can be sourced from the inside tissues of wood.
Lignin hardens when mixed with non-toxic ingredients. This is a breakthrough in man’s search for plastic replacement. Plastic is harmful to the environment while this new liquid wood is environment friendly, bio-degradable and can adapt the many applications and uses of plastic.
Experiments on liquid wood first came to fruition at the Institute for Chemical Technology in Germany. Lignin, a component of wood, was mixed with several organic materials and liquid wood was born. The mixture of lignin with other materials allowed it to be moulded into the size or form desired.
Arboform is still wood but has the qualities of plastic in that it can be moulded into different forms. Arboform has already been applied commercially with the production of car components. More modifications and experiments are still conducted on arboform. (Urushev, 2009)
Is there a more environmentally sustainable material and process?
Manufacturers have been trying to create products that have less environmental impact. Sustainable materials are those that have enough supply.
Some techniques and solutions include the 3Rs – recycle, reduce and reuse. Others include similar moves like recovery and one of the most important is organic recycling which is also known as composting. These are alternative methods that are environmentally friendly.
Plastic wastes can also be reverted back into their chemical composition. But this has to anticipate biodegradation where molecules of the plastic haven’t changed. Degradation in plastics occurs after so many years. They are so-called non-biodegradable materials which are harmful to the environment.
The invention of liquid wood to replace plastic is the most likely recommendation that this paper can offer for an environmentally friendly materials in our homes, offices, and places of work.
References
Green, M. and Wittcoff, H., 2003. Organic chemistry principles and industrial practice. Germany: WILEY-VCH Verlag GmbH & Co. KGaA, Winheim.
Patrick, S., 2005. Practical guide to polyvinyl chloride. UK: Rapra Technology Ltd.
Thornton, J., 2000. Pandora’s poison: chlorine, health, and a new environmental strategy. United States of America: Massachusetts Institute of Technology.
Tripathi, D., 2002. Practical guide to polypropylene. UK: Rapra Technology Ltd.
Urushev, P., 2009. Liquid wood to replace plastic. Available from: http://www.pravdareport.com/science/106988-liquid_wood/
Vasile, C. and Pascu, M., 2005. Practical guide to polyethylene. UK: Rapra Technology Limited.
Wittcoff, H., 2004. Industrial organic chemicals. New Jersey and Canada: John Wiley & Sons, Inc.
Wittcoff, H. et al., 2004. Industrial organic chemicals. New Jersey and Canada: John Wiley & Sons, Inc.
Wunsch, J. R., 2000. Polystyrene – Synthesis, production and applications. UK: Rapra Technology Ltd.
Zweifel, H., 2009. Plastics additives handbook. United States and Canada: Hanser Publications.