Objectives
Identify types of intermolecular forces that are present in molecules.
Describe how intermolecular forces occur in different molecules.
Compare the strengths of different types of intermolecular forces in molecules based on their boiling points.
Chemical concepts required as prior knowledge
Molecules, intermolecular forces, polar molecules, non-polar molecules, boiling points, and electrostatic forces.
Models of intermolecular forces
The concepts of intermolecular forces used in the development of models emanated from numerous sources (Pauling 374; Rummens 12; Stone 5). These sources define and illustrate the relationships among different types of intermolecular forces. The models offer important information about the intermolecular forces, specifically van der Waals forces.
Model 1: Showing types of intermolecular forces and describing their occurrence.
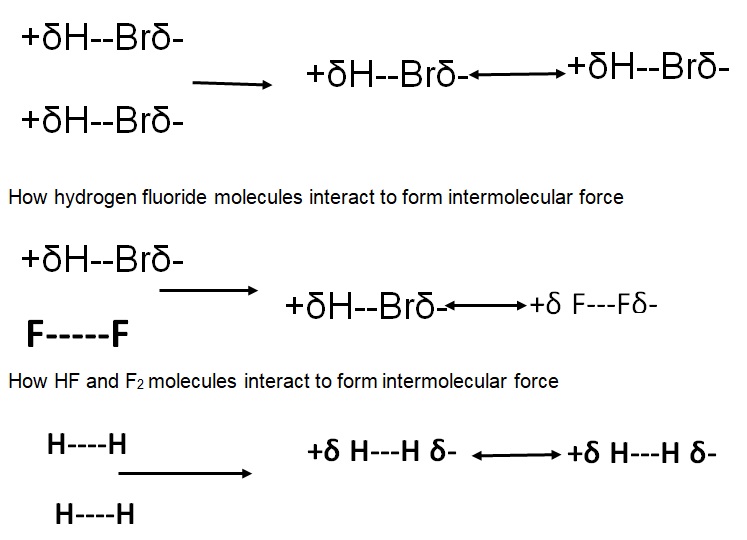
How H2 molecules interact to form intermolecular force.
Exploration Questions
- What are the molecules that the model uses in illustrating intermolecular forces?
- From the model, where does intermolecular force occur?
- What is the difference between HBr and H2 molecules in the model?
Concept Invention Questions
- How do HBr molecules interact to form intermolecular forces?
- How do HBr and F2 molecules interact to form intermolecular force?
- How do H2 molecules interact to form intermolecular force?
Application Questions
- What is the difference between F2 before and after interaction to form intermolecular force?
- In the model, which are the polar ends of the molecule?
- What determines polarity of a molecule when molecules interact?
Divergent Question
- Which are the molecules that would have the same intermolecular forces as the model illustrates?
Model 2: Showing properties of intermolecular bonds in terms of strengths of bonds and boiling point trends.
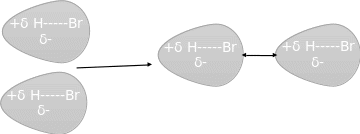
Intermolecular force between two polar molecules e.g. HBr/
Exploration Questions
- What are the types of intermolecular forces that the model illustrates?
- By comparing boiling points, what is the hierarchy of intermolecular forces’ strengths from the weakest to the strongest?
- Which is an intermolecular force has temporary attractive forces?
Concept Invention Questions
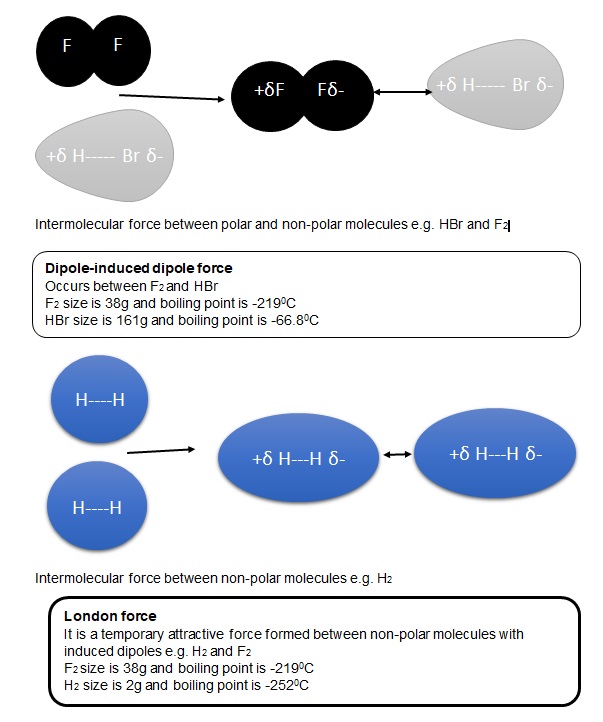
- How does dipole-induced dipole force occur in molecules?
- How does dipole-dipole occur in molecules?
- What is an appropriate description of London forces?
Application Questions
- How do non-polar molecules acquire dipoles and start forming London forces?
- How does polarity influence strength of intermolecular force and boiling point?
- What is the trend of boiling points across the molecules with the types of intermolecular forces illustrated in the model?
Divergent Question
- What are the examples of molecules in each type of intermolecular that the model illustrates?
Learning Cycle
The learning cycle is an effective method of teaching science because it is the basis of systematic inquiry-based approach. Teaching and learning of science require a systematic approach, which allows learners to advance their learning process by making inquiries about a given phenomenon. The learning cycle comprises three phases, namely, the exploration phase, the concept invention phase, and the concept application phase. These phases closely interrelate and form a process of learning where learners explore a phenomenon of interest, invent concepts, and apply invented concepts in elucidating a scientific phenomenon. Essentially, the learning cycle indicates that learning is a continuous process.
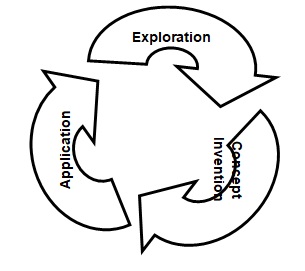
The exploration is the first phase of the learning cycle, which presents learners with a new scientific phenomenon so that they can have firsthand experience. In the exploration phase, learners explore a scientific phenomenon and familiarize themselves with the concepts that it entails (Zubrowski 44). In the POGIL activity of the intermolecular forces, the exploration questions enable learners to explore intermolecular forces using prior knowledge. The exploration questions revolve around molecules, ions, intermolecular forces, ionic forces, dipole forces, ion-dipole forces, London forces, and van der Waals forces amongst others. With the help of their instructors, learners can effectively explore what constitutes intermolecular forces.
The concept invention is the second phase of the learning cycle that enables learners to conceptualize a scientific phenomenon by interacting with instructors, peers, and concept maps. Fundamentally, concept invention entails creating relationships of diverse concepts and visualizing a whole phenomenon in a concept map (Bybee 53). The purpose of the concept invention is to allow learners to prove their hypotheses and formulate theories, which they can apply in scientific studies regarding a phenomenon of interest. In the POGIL activity, instructors should enable learners to conceptualize the relationships between diverse types of intermolecular forces using a concept map, which effectively elucidates the nature and existence of these forces. The questions of the concept invention provided enable learners to conceptualize intermolecular forces in ions and molecules.
The concept application is the third phase of the learning cycle that allows learners to apply their concepts and concept map in diverse phenomena. In the concept application, instructions assess knowledge and skills that learners gained during the phases of exploration and concept invention by presenting new situations or availing related scientific phenomena (Ornek and Saleh 214). The application questions in the POGIL activity effectively assess the knowledge and skills of learners because it covers considerable aspects of intermolecular forces. At the end of this phase, learners should have achieved all objectives of the POGIL activity, which are to identify types of intermolecular forces that are present in ions and molecules, differentiate intermolecular forces that are in ions and molecules, and construct a concept map showing the interrelationships of diverse intermolecular forces. In this view, the phase of concept application plays a central in the POGIL activity because it sets a platform for the next cycle of learning where learners explore and conceptualize a phenomenon, and subsequently apply knowledge and skills acquired in the process of learning.
Works Cited
Bybee, Rodger. Learning Science and the Science of Learning: Science Educators’ Essay Collection. Arlington: National Science Teachers Association Press, 2002. Print.
Ornek, Funda, and Issa Saleh. Contemporary Science Teaching Approaches: Promoting Conceptual Understanding in Science. Charlotte: Information Age Pub, 2012. Print.
Pauling, Linus. General Chemistry. New York: Oxford University Press, 2014. Print.
Rummens, Frans. Van der Waals Forces and Shielding Effects. New York: Springer cience & Mass Media, 2013. Print.
Stone, Anthony. The Theory of Intermolecular Forces. New York: Oxford University Press, 2013. Print.
Zubrowski, Bernard. Exploration and Meaning Making in the Learning of Science. Dordrecht: Springer, 2009. Print.