Overview of ankle replacement technologies
The ankle joint is complex in that it involves four structures: the lower end and medial malleolus of the tibia and the lateral malleolus of the fibula and the trochlear surface of the talus. This joint resembles a mortise-and-tenon joint used in carpentry (Banks, McGlammy and Martin 21). The tibia and fibula must be bound together for the mortise to be stable. This is done by the syndesmosis, which consists of the anterior tibiofibular ligament, interosseous ligament, and posterior tibiofibular ligament (Bolton-Maggs, Freeman and Suddow 785).
Interest in ankle replacement technologies has risen due to the rise in the number of rheumatoid arthritis patients. The major cause of ankle arthritis is the trauma of the ankle, followed by inflammatory arthropathies and structural deformity (Bhandari 126). The ankle joint is resilient to the process of aging and primary osteoarthritis (Bhandari 126).
The ankle acts mainly as a hinge joint, allowing plantarflexion and dorsiflexion. Instability of this joint could lead to degenerative changes of the joint. It is strengthened on the medial side by the triangular deltoid ligament, which radiates from the medial border of the plantar calcaneonavicular ligament, the tuberosity of the navicular, and the neck of the talus (Kurtz 65). The lateral collateral ligament consists of the anterior and posterior tibiofibular ligaments and a calcaneofibular ligament. These structures are essential for the accurate functioning and stability of the joint (Easlay 301).
There is a lot of evidence to show that ankle replacement surgery is riddled with many complications. In 2006 Scuberth published the perioperative complications rate of the first 50 consecutive total ankle replacements performed by a single surgeon (Bhandari 126). There was a 38% rate of interoperative malleolar fractures, 24% occurrence of some degree of component malalignment, 18% incidence of minor wound healing disturbances that resolved with local wound care, and a 2% rate of major wound complications requiring flap coverage (Bhandari 77).
An early component revision was required in 16% and each of these complications, other than wound complications, decreased with surgeon experience. An evidence-based classification system for complications in ankle replacement had proposed. This system classifies complications according to their potential to cause the failure of the prosthesis (or removal of implants). Deep infection, aseptic loosening, and implant failure were classified as high-grade complications since they were shown to result in failure greater than 50% of the time (Bhandari 77). Technical errors, subsidence, and postoperative bone fracture were classified as medium-grade resulting in failure less than 50% of the time. Intraoperative bone fractures and wound healing problems were classified as low grade resulting in failure less than 50% of the time (Bhandari 78).
It is however recommended that supplementary extra-articular realignment procedures be performed concomitantly with total ankle replacement since they are not associated with an increased incidence of complications (Bhandari 79). In general, a surgeon can experience 20 to 60% complications, although most of these do not pose a threat to the longevity of the prosthesis itself (Bhandari 79).
The workings of the ankle joint
The ankle joint is a union of three bones: the tibia, the fibula, and the talus of the foot (Bolton-Maggs, Freeman and Suddow 786). The ankle is responsible for load-bearing and kinematic functions. The anatomical configuration of the ankle joints is similar to that of the hip (Bolton-Maggs, Freeman and Suddow 786). The ankle joint is inherently more stable than the knee joint, which requires ligamentous and muscular restraints for its stability.
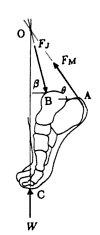
W– The force applied on the foot as the ground reaction force, FM is the magnitude of the tensile force of the gastrocnemius and soleus muscles on the calcaneus through the achilles tendon, and Fj is the magnitude of the ankle joint reaction force applied by the tibia on the dome of the talus. The achilles tendon is attached to the calcaneus at A, the ankle joint center is located at B, and the ground reaction force is applied on the foot at C.
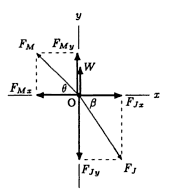
There are three-force systems composed of the muscle force, Fm, joint reaction force, Fl, and the ground reaction force W. The forces acting on the foot do not form a parallel force system (Ozkaya, Nordin and Leger 112).
In the mathematical model of the foot motion equations of motion must be known and applied well (Banks, McGlammy and Martin 127). In this case, a small portion of the gait cycle is taken into consideration. This is usually from heel strike to toe slap because this is the portion that is not functioning well in the patient with ankle-joint arthritis and is supported by the ankle brace (Banks, McGlammy and Martin 127).
Also, the foot is considered to be a single degree of freedom (SDOF) oscillator, pinned at the contact point with the ground (heel) and is able to rotate in the sagittal plane (side view). The equation of motion for an SDOF oscillator is given as:
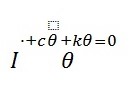
Equation 1, is presented in terms of damping coefficient and natural frequency and the parameters that are easier to specify are as follows (Weiss, Hunter and and Kearney 539):
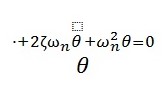
This equation can then be applied to determine the motion of the foot by summing up the moments about the heel using variables in figure 3.
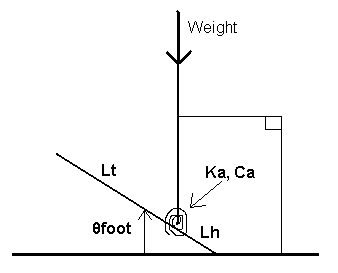
In Figure 3; Lh is the distance from heel to ankle, Lt is the length from ankle to toes, Ka and Ca are the stiffness and damping of the ankle, respectively. The angle of the foot above the ground (θfoot) and the downward force of weight are represented by the patient’s body mass. The mass moments of inertia IA and IF, taken about the ankle and the heel, respectively, are used (Weiss, Hunter and and Kearney 541). In the ankle replacement, these forces and dimensions are important in maintaining stability and flexibility of the body.
Advances in ankle replacement designs
The goal of ankle replacement design is to mimic the ankle joint as closely as possible. Specifically, a suitable range of motion should be available to allow for proper gait patterns and other activities of daily living. Total ankle replacement designs are expected to have a reproducible surgical technique, minimal bone resection, rapid and adequate bone in-growth, minimal constraint and replication of physiological ankle motion, and pain relief (Bolton-Maggs, Freeman and Suddow 788). The design should offer minimal complications and need for early revision, as well as long-term survivorship. The contact area should be appropriate to distribute the load and avoid high stresses (Bolton-Maggs, Freeman and Suddow 788).
The first total ankle replacement was designed in 1970 and implanted by Lord and Marotte (Kurtz 65). The design contained a tibial component with a long stem that was similar to the femoral component with a long stem that was similar to the femoral component of total hip replacements. Overall, indications for the initial TAR implants were limited due to the high complication rates and failure, with recommendations for use only in elderly patients with limited physical demands (Kurtz 66).
Early designs recognized that the use of cement required larger resection of bone, which could result in subsidence of the metal components. In considering materials for the design it is important that ability of the biomaterial to support the total body weight is taken into considerations.
Also, there was a need to strike a balance between stability of the prosthesis with achieving larger range of motion. The results of the Marotte and Lord prosthesis were poor, with a high rate of secondary varus instability, and the design was short-lived.
The Roy-Camille prosthesis was based upon the same principle, and equally fraught with a high rate of early implant failures. The main problem with this design also was hind-foot tilt into varus (Bouysset, Tourne’ and Tillmann 97). In 1972, Tomeno and Cornic described a sliding cylinder-type device, without mortise extensions; the talar component was made of metal, and the tibial component of PE. In the same year, Freeman proposed a more constrained cylindrical pattern, with flanges (Bouysset, Tourne’ and Tillmann 97).
The FDA currently has three classifications for TARs, tow of which are Class II (metal/polymer or metal/composite semi-constrained cement prosthesis) and one that is class III (non-constrained cemented prosthesis). There are currently 20 different TAAR designs worldwide that have been released for clinical use or are currently in development. FDA clearance (Class II) is currently limited to the Eclipse Total Ankle Implant (Kinetikos Medical, Inc), Salto TalarisTM Total Ankle Prosthesis (Tornier), Agility Total Ankle Prosthesis (Depuy Orthopaedics), INBONE Total Ankle (INBONE Technologies, formerly Topez Orthopaedics), and the Brigham Total Ankle Prosthesis (Howmedica Corp.). To date there are no FDA- approved, non-constrained (Class III) TARs, although an FDA panel recommended approval of the STAR device in April 2007, with final approval of the device pending (Easlay 263).
Reasons for ankle replacement
Degenerative change of the ankle occurs either after a fracture or after ligamentous instability. Only a few cases are truly idiopathic. The post-fracture group could be divided into two groups. The first comprises patients with severe soft tissue injury, high-energy injury and multiple operated tibial pilon (Bhandari 80). These patients usually have a compromised, scarred sift tissue envelope, and the ankle has limited motion. Pain is due to the ankle arthritis but also the soft tissue problems, including scar and damaged lymphatic and venous flow. The second group comprises patients with simple malleolar fractures, low energy pilon with minimal soft tissue compromise. This group behaves more like the ligament instability or idiopathic group in that the soft tissues are friendly and the ankle range of motion is generally very well preserved (Bhandari 81). The instability group could have additional issues, including peroneal tendinosis or rupture as well as secondary sub-talar arthritis or hind-foot varus.
Specifications for ankle replacement
Total ankle replacement (TAR) prostheses is used to provide functioning joint connection by use of talar and tibial components that allow for a minimum of 150 of dorsiflexion and 150 to 250 of plantar flexion, as determined by non-clinical testing.
Biomaterials are the building blocks of implant technology. They may be formally defined as natural or synthetic materials that replace or supplement the function of biologic tissues in the human body (Banks, McGlammy and Martin 127). Their primary function is often to provide structural support. In reconstructive surgery of the ankle, it is often that biomaterials possess the mechanical characteristics necessary to withstand forces and motions experienced by the normal tissue they are to replace.
Physical characteristics of implant materials such as firmness, tensile strength, and surface characteristics play an important role in structural integrity (Banks, McGlammy and Martin 127). However, the new material must first be tested for biocompatibility and found to be safe before a specific implant configuration could be evaluated. The following are specifications for an ideal implant material that was proposed as early as 1953 (Banks, McGlammy and Martin 128):
- Should resist change by the body tissues
- Should not react with body tissues (that is inert)
- Not eliciting an inflammatory or foreign response
- Noncarcinogenic
- Nonallergenic
- Structurally stable
- Capable of fabrication in the forms desired
- Capable of being sterilized
It is also more desirable that an implant to undergo gradual biodegradation or solely serve as a temporary scaffold, thereby allowing new bone growth or encouraging tissue regeneration, rather than acting as a permanent tissue replacement (Banks, McGlammy and Martin 128).
Surgical management of ankle replacement
The operation starts by making an incision through the front skin of the ankle in a process termed as anterior approach (Easlay 302 ). The nerves, tendons and blood vessels are protected and moved to the side. The ankle joint is entered by making “an incision into the joint capsule that surrounds the ankle joint” (Easlay 303). A bone or a biomaterial of required characteristics is prepared to replace the ankle joint surfaces.
In the next step, “the bones that make up the socket of the ankle joint (tibia and the fibula) are cut so that the metal socket or biomaterial can fit into the place” (Easlay 302). “The top of the talus is removed so that the metal talus component can be inserted” (Easlay 302). Then all “the tibial implant and the talar implant portions of the artificial ankle joint are then inserted” (Easlay 303). Bone grafts are then placed between the fibula and the tibia “to ensure that fusion between the two bones takes place” and as a consequences, “this stops the motion between the two bones that could loosen the artificial joint” (Easlay 303).
The ankle is tested to make sure that the pieces fit properly “to ensure that the ankle socket or the mortise components fits tightly and two screws” are placed between the fibula and the tibia just above the ankle joint (Easlay 302). After successful fitting and fixing during the surgery operation, the ankle joint capsule is fixed back followed by the skin fixing. A large bandage and a support material “are placed on the lower leg to protect the new ankle joint as the leg heals” (Easlay 302).
Works Cited
Banks, Alans S, et al. McGlammy’s Comprehensive Textbook of Foot and Ankle Surgery Vol 2. Philadelphia: Lippincott Williams and Wilkins, 2001.
Bhandari, Mohit. Evidence-based Orthopedics. West Sussex: Wiley-Blackwell Publishing Ltd, 2008.
Bolton-Maggs, BG, MA Freeman and RA Suddow. “Total ankle arthroplasty. A long-term review of the London Hospital Experience.” Journal of Bone and Joint Surgery of Britain, (1985); 67: 785-790.
Bouysset, Maurice, Yves Tourne’ and Karl Tillmann. Foot and Ankle in Rheumatoid Arthritis. France: Springer Verlag, 2006.
Easlay, Mark E. Operative Techniques in foot and ankle surgery. Boston: Lippincott Williams and Wilkins, 2010.
Kurtz, Steven M. UHMWPE: Biomaterials Handbook. Massachusett: Elsevier Academic Press, 2009.
Ozkaya, Nikat, Margareta Nordin and Dawn Leger. Fundamentals of biomechanics: equilibrium, motion and deformation. New York: Springer Sciences Inc, 1998.
Weiss, P.L., I.W. Hunter and R.E and Kearney. “Human Ankle Joint Stiffness Over the Full Range of Muscle Activation Levels.” Journal of Biomechanics (2004): 539-544.