Introduction
The Bratter’s Syndrome consists of a group of almost similar kidney disorders that result in an imbalance of “potassium, sodium, chloride, and related molecules in the body” (Nooh, Abdullah and Sheta 419). The condition is rare, but as a significant congenital type of secondary hyperaldosteronism because of inability of the renal system to control electrolytes. Bratter’s Syndrome may be depicted through hyperplasia (unusual growth of cells) and hypertrophy (enlarged cell growth) of the juxtaglomerular cells. It presents normal blood pressure without observed edema. In some instances, Bratter’s Syndrome may be noticeable before birth due to the presence of polyhydramnios.
This is apparent from the “increased volume of amniotic fluid surrounding the fetus” (Colussi 3). The increased volume of amniotic fluid is “most likely to cause premature birth” (Colussi 3). Children with Bratter’s Syndrome normally fail to thrive, i.e., grow as expected.
The condition results in excessive loss of salt through urine. The loss of salt (sodium) causes “constipation, dehydration and excessive production of urine or polyuria” (Nooh et al. 419). This situation results in weaker bones (osteopenia). Further, calcium deposits may be found in “the kidney, leading to hardening of the kidney, a condition known as nephrocalcinosis” (Nooh et al. 419). The condition also causes the level of potassium to drop in the blood. Hypokalemia is responsible for weak muscles, fatigue and cramping in individuals with the disorder (Nooh et al. 419).
In rear cases, individuals with the disorder may experience loss of hearing due to abnormalities within the inner ear. Different types of Bratter’s Syndrome may be identified by the time of onset and severity. The severe form normally starts before birth and it could be life threatening. The classical type starts during early periods of childhood and is not severe relative to the other one. It was established that Bratter’s Syndrome is associated with genetic disorder. On this regard, several other types of the Syndrome have been identified, including type I, II and IV, which are associated with conditions before birth while type IV may be linked to loss of hearing. The third type reflects classical features of Bratter’s Syndrome.
How Bartter Syndrome affects the function of the kidney
Among the many roles that the kidney plays in the body, the most critical one is the control of the composition and the fluid volume in the body (Colussi 2). The kidney does this role by filtering blood at glomeruli and then reabsorbs the required constituents from the fluid. Various distinct parts of the renal tubules conduct these functions to ensure normal body fluid composition is maintained. Failure to perform any of these functions could result in many severe consequences for the body. Such consequences could result in death.
Bratter’s Syndrome impairs kidney’s function. The genetic defects are responsible for impairing normal functions particularly the protein that is responsible for reabsorption of chloride and sodium in certain areas of the renal tubule, the Henle loop. The disorder may also affect reabsorption of other elements such as “potassium, calcium, magnesium (Mg) and water either directly or indirectly” (Colussi 2). The Henle loop is responsible for reabsorption of significant amount of sodium and chloride and therefore any form of impairment is detrimental to the normal functions of the body.
It is imperative to recognize that the extent of the impairment may differ from one patient to another and hence clinical symptoms may differ based on the form of severity. The first notable consequence of the Bratter’s Syndrome is increased volume of urine. The known symptoms of the disease include frequent urination, the necessity to get up often and several cases of bed-wetting (nocturnal enuresis) until later stages of adolescence (Colussi 2).
The disorder is associated with abnormal loss of water and therefore affected persons are normally polydipsic. Too much urine from the fetus may cause pregnancy complications and premature delivery.
The function of a normal kidney compared to a patient’s kidney suffering from Bratter’s syndrome
A normal kidney filters out waste materials, excess water and salts from the blood to maintain optimal conditions. Most of these activities take place at the proximal and distal glomorulous, the loop of Henle and in the collecting duct. The glomulerous maintains constant filtration while the tubule ensures that the kidney maintains constant regulation of the body fluids.
Bratter’s Syndrome, however, impairs functions of the normal kidney, causing cases of mild to severe conditions. Once the ascending Henle loop is impaired, the kidney cannot function normally, leading to chronic kidney failure and ultimately death if no effective treatment, which is kidney transplantation, is performed.
Using implantable bioartificial kidney as a therapy to replace the function of a normal kidney
A severe case of Bratter’s Syndrome leads to chronic kidney disease or end stage renal disease, which slow, progressive loss of kidney activities (UCSF 1). A near-complete-to-total kidney “failure leads to ESRD” (UCSF 1). Consequently, the kidney is unable to perform its regular activities, which are necessary for sustaining life. Accumulation of naturally occurring metabolic byproduct and excess water and salts due to failed kidney could be detrimental to any person. In addition, the kidney may not be able to replenish sufficient amount of electrolyte elements in the blood and production of life-sustaining hormones.
It is imperative to note that the kidney plays significant and diverse roles in the body. Hence, many patients with cases of kidney failure experience extreme cases of illness alongside cardiovascular complications and infections. Without effective interventions, individuals with the end stage renal disease are not likely to live long. Such patients may depend on short-term measures such as dialysis to prolong their lives.
Alternatively, kidney transplantation is available as a long-term solution to chronic cases. In this regard, kidney transplantation is the best option for many patients. However, there is a widespread shortage of donor organs to meet rising demands for kidney among patients and therefore many patients are not able to find donors. These forms of interventions also have severe drawbacks (UCSF 1). Patients with ESRD experience “anxiety while the condition causes huge toll on other members of their families” (UCSF 1). Unfortunately, the number of patients with chronic cases of kidney disorder continues to rise globally, causing a strain on few available donated organs. Hence, there is a need for new forms of treatment options.
The end stage renal disease requires efficient, patient-oriented, stable and cost-effective interventions. Such interventions should restore functional abilities of a normal kidney to counteract inadequate supply of donor organs.
Initially, dialysis was the first intervention for the chronic kidney disease and then came the kidney transplant. Since then, researchers have made significant advances to find suitable solutions for such patients, including improvements on existing treatments (Cieslinski and Humes 678-681). However, minimal improvements have been made. It is believed that emerging technologies could help in developing the third treatment option for the ESRD.
Before the invention of a new treatment option for ESRD or chronic condition, researchers have made significant progresses toward identifying the best way technologies and science can interact to develop artificial kidney or parts of it that can mimic endocrine, metabolic, immunological roles of a normal kidney (UCSF 1). As researchers continue to make advances, previous challenges are now being solved.
For instance, the current silicon nanotechnology has ensured that vital parts for producing reliable membranes can now be done in mass. New forms of molecular coatings have ensured that silicon is now compatible with blood and therefore can protect the membrane without any functional technicalities (UCSF 1). Improvements in “cell sourcing and storage have provided solutions for readily available cells at the right state” (UCSF 1).
As a result, academics, engineers, scientists and physicians have continued to focus on these new opportunities to introduce a new form of ESRD treatment. The new option promises an effective, affordable ESRD treatment option because of rising cases of chronic kidney conditions.
The University of California, San Francisco (UCSF) has focused on a kidney project that can be “surgically implanted, free-standing bioartificial kidney to perform the vast majority of the filtration, balancing and other biological functions of the natural kidney” (UCSF 1). The artificial kidney consists of two parts, which are possible because of recent developments in “silicon nanotechnology, cell science, and membrane filtration” (UCSF 1). It will rely on individual’s blood pressure and therefore no need for “tethers and external tubes or immunosuppressant drugs” (UCSF 1). As the institution and researchers focus on the new ESRD treatment option, the device continues to make progress and the first-in-human clinical trials are anticipated by the year 2017 (UCSF 1).
Current studies in bioartificial kidney have established improvements. Salvatori et al. (379) note that collaborative efforts would result in bioengineered kidney with capabilities of providing renal functions for ESRD patients. These new devices could be wearable or implantable to replace impaired kidney completely and reduce morbidity and mortality in patients with ESRD (Humes et al. 343). Oo et al. (497) have shown that the novel bioartificial kidney design will enhance safety and performance and lead to improved comprehension of effects of these devices.
How the implantable bioartificial kidney should look like
A bioartificial kidney for chronic renal failure is expected to look like the actual kidney. However, the implantable bioartificial kidney looks completely different from the actual kidney. Nevertheless, the device is designed to perform in a similar manner as a real human kidney. It will extract wastes and maintain the required normal body nutrients (Bole 1).
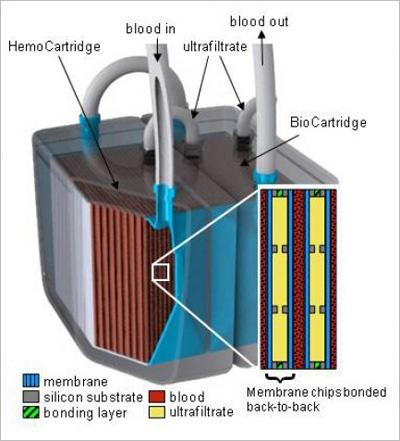
Composition of the implantable bioartificial kidney
The bioartificial kidney device from the UCSF would have several microscopic filters and a bioreactor of renal cells to ensure that it can replicate “the metabolic and water-balancing roles of a real kidney” (Cisneros and Bole 1). It consists of hollow-like fibers from polymer membranes with pores that range from 5 to 90 nm silicon nanotechnology used design the slit pore membrane. The bioartificial kidney also has microelectromechanical systems and sieving coefficient. In addition, it does not require any external power source, but instead relies on the blood pressure. No cases of blood clotting expected because it is high quality device.
The bioartificial kidney will fulfill two roles, namely filtration and regulation of the body fluid. The available intervention has failed to serve regulatory function. The design will be implanted and therefore no patient will be tethered on a machine. This would enhance the quality of a patient’s life.
The bioartificial kidney will be miniaturized with nanopores developed by microelectromechanical systems. It will achieve reduced sieving coefficient because of the silicon nanopores.
Although a bioartificial kidney presents an opportunity for patients, its developers must ensure that it can contain adequate fluid volume to ensure effective dialytic therapy. Another shortcoming involves conducting invasive surgical techniques for the replacement and implant relative to WEBAK. Overall, costs could encourage many patients that require bioartificial kidney implantation because it is relatively cheaper when compared to hemodialysis costs.
How implantable bioartificial kidney will work
The bioartificial kidney will fulfill two roles, namely filtration and regulation of the body fluid. Currently, an external model has been used to treat an end stage renal disease in extremely sick patient successfully (UCSF 1). With further improvements, it would be easier to implant the device, allowing the patient to live a more regular life (UCSF 1).
Conclusion
Bratter’s Syndrome is a “rare genetic condition that affects the normal functions of the kidney” (Nooh et al. 419). Severe cases of the disorder render the kidney ineffective, leading to kidney failure, which progresses to chronic condition and end stage renal disease. Dialysis provides short-term relief while a long-term solution from kidney transplant is rare because of few donated organs. Hence, the third treatment option can only be found in a bioartificial kidney device. Further studies are expected to identify and resolve any potential challenges with the device.
Works Cited
Bole, Kristen. UCSF Artificial Kidney Project Tapped for Accelerated FDA Program. 2012. Web.
Cieslinski, Deborah A and David Humes. “Tissue Engineering of a Bioartificial Kidney.” Biotechnology and Bioengineering 43 (1994): 678-681.
Cisneros, Lisa and Kristen Bole. Artificial Kidney Holds Promise for Vast Majority on Dialysis. 2013. Web.
Colussi, Giacomo. “Bartter syndrome.” Orphanet Encyclopedia. 2005. Web.
Humes, H. David, Deborah Buffington, Angela J. Westover, Shuvo Roy, William H Fissell. “The bioartificial kidney: current status and future promise.” Pediatric Nephrology 29.3 (2013): 343-351. Print.
Nooh, Nasser, Walid Abdullah and Saad Sheta. “Anesthetic management of a patient with Bartter’s syndrome undergoing bilateral sagittal split osteotomy.” Saudi Journal of Anaesthetic 6.4 (2012): 419–422. Print.
Oo, Zay Yar, Karthikeyan Kandasamy, Farah Tasnim, Daniele Zink. “A novel design of bioartificial kidneys with improved cell performance and haemocompatibility.” Journal of Cellular and Molecular Medicine 17.4 (2013): 497–507. Print.
Salvatori, Marcus, Andrea Peloso, Ravi Katari, Giuseppe Orlando. “Regeneration and Bioengineering of the Kidney: Current Status and Future Challenges.” Current Urology Reports 15.1 (2014): 379. Print.
UCSF. The Kidney Project: Creating a bioartificial kidney as a permanent solution to end stage renal disease. 2015. Web.