Popular Article
Shuttle, A. (2020). A new kind of battery that removes carbon dioxide from the air? Sciworthy.
Main Points
The article describes a novel, potentially scalable carbon capture method that can help cut industrial greenhouse gas emissions. A key point addressed is the climate change effects of fossil fuel combustion that can be mitigated through carbon dioxide (CO2) sequestration technologies. Existing methods are difficult to scale, energy inefficient, and designed for emissions with over 10% CO2 levels (Shuttle, 2020). The authors describe a battery built using a new technology – electro-swing-adsorption (ESA) – that traps and releases pure CO2 for storage in underground systems or reuse in other applications such as greenhouses. They explain that the device’s anode and cathode are built from carbon nanotubes and polymerized with quinone molecules, which, through the charge or discharge process, adsorb CO2 when reduced and release it when oxidized. The unique aspects of this battery include it is energy-efficient, durable, and scalable, can absorb or release trapped CO2 for storage, and economically feasible.
Background Information
Knowledge of greenhouse gases and how they trap solar radiation, making the planet habitable for all life, is required to understand this article. Background information by the reader about the human activities that cause shifts in atmospheric CO2, affecting climate and weather patterns, is also crucial. Knowledge of climate change-related increasing global temperatures, polar ice cap melting, catastrophic storms, wildlife species decline, and rising sea levels would help understand the need for carbon capture initiatives, including the new technology described in the article.
Information
The popular press article outlined the title of the original study reviewed and the authors’ names at the end. Also included were the institution to which the researchers are affiliated and the funding organization. The article also directly referred to the authors and the electro-swing absorption technology for carbon capture. With this information, it was possible to search the original scientific article on the web to further analyze the topic.
Additional Information
The article describes the battery’s carbon capture mechanism as an oxidation/reduction cycle that involves an alternating electrode charge or discharge process. I would like to see how gaining two electrons during reduction increases the selective absorptive capacity of the polyathraquinone cathode on charging. How is oxygen absorption by the quinone molecules avoided in this redox reaction? Also, the regeneration of the bound CO2 requires further explanation. Indicating the quinone intermediates formed by adding CO2 (carboxylation) would be useful. Does increasing the voltage level elevate the amount of CO2 produced by the battery? The authors state that the economic feasibility of the method has been demonstrated through financial analysis. According to Shuttle (2020), it would cost $50-$100 to sequester one ton of CO2 using the novel technique. However, cost-benefit analysis and comparisons with other technologies to establish the new method’s superiority are lacking.
Author Credibility
The author of the article is a qualified and credible writer in the carbon capture research field. From his LinkedIn profile, Adam Shuttle is a material science researcher focusing on electrocatalysis and related green solutions and writes on his sustainability blog. Professionally, he has demonstrated laboratory experience, engaging in associate research with the Blue Marble Space Institute. He studies a Master’s in Materials Science at the University of Oxford. Given his professional background and research interest in sustainable technologies, I think the author was not biased but rather objective in reporting the new carbon capture technique. His article gives a balanced picture of the technology’s CO2 adsorption and release mechanism.
Scholarly Article
Voskian, S., & Hatton, T. A. (2019). Faradaic electro-swing reactive adsorption for CO2 capture. Energy & Environmental Science, 12, 3530-3547.
Hypothesis
The study tested the hypothesis that electrochemically charged parallel stacked systems with airflow channels can trap CO2 in a stream of exhaust gas or industrial fumes. Another premise included in the article is that reversing an ESA’s polarity would lead to CO2 release in a mechanism analogous to temperature- and pressure-swing adsorption (TSA and PSA) operations (Voskian & Hatton, 2019). However, a null hypothesis is not indicated in this study.
Type of Study
The authors used an experimental design to test the research hypothesis. Their approach entails describing the study materials, including the dichloro-1,4-anthraquinone polymer used in the CO2 capture and release technique. The sampling technique (no participants involved) is not stated,eight8-bed but the suppliers and specific reagent preparation steps are provided. The study involved a laboratory experiment type, which ensured accurate measurements of the variables under controlled conditions.
Benefits and Drawbacks
A laboratory experiment has advantages over other study designs and approaches. The highly controlled environment allows the researcher to determine cause-effect relationships accurately – confounding effects and bias can be removed. Additionally, the internal validity of experimental research is high due to the robust design. A major drawback of this method is the limited external validity of these studies when human subjects are involved. People are likely to behave differently in real life versus laboratory settings, making it difficult to generalize results. Also, ethical concerns limit the usefulness of experimental designs involving humans.
Variables Measured
Electrochemical measurements were conducted to determine the effectiveness of the electrodes in trapping CO2. In this regard, the researchers measured two polyvinylferrocene-carbon nanotubes (PVFc-CNT) current and potential (voltage) or CV and CO2 moles captured and released per cycle (dependent variables) by nitrogen- or CO2-saturated (independent variables) PVFc-CNT electrodes. Pressure changes in the electrochemical chamber were also measured as an effect of CO2 adsorption and desorption.
Sample Size
The research tested a CO2 capture and release system using gas mixtures (carbon dioxide and nitrogen). As the study was a lab experiment, no human or animal subjects were sampled. Therefore, determining whether or not the sample size is a good representation of the larger population may not be practical for the research review. However, adequate measurements of the variables – nitrogen and CO2 saturation – were made to assess the postulated effect.
Assumptions
The experiment followed standard procedures and tests and had some technical assumptions. The PvFc-CNT anode was separately saturated with N2 and CO2, as it was assumed that a difference in the current response would be detected. An assumption made when measuring the rate of CO2 adsorption was that the charging of quinones was in balance with CO2 levels at any point of the charging process.
Use of Statistics
Measurement data were used to determine the efficacy of the CO2 capture and release technology. The statistical test used in analyzing the adsorptive effectiveness of the system was the mass transfer governing equations. Current results were modeled at three CO2 levels of 0, 2, and 5% to determine the optimal charging method (Voskian & Hatton, 2019). Figure 1 below shows the simulated CO2 concentration (50%) profiles, with optimal adsorption, reached 8-bed volumes.
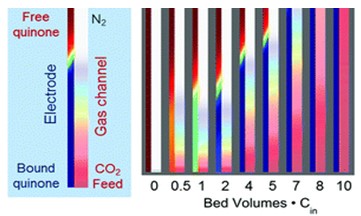
Main Conclusions
The authors conclude that ESA can mediate carboxylation, trapping CO2 in emissions through redox-active molecules. Their main findings are that the technique effectively absorbs CO2 at concentrations found in fuel combustion fumes (around 10%), and it can reach 7000 adsorption cycles at minimal energy (Voskian & Hatton, 2019). The implication is that incorporating the new electrode compounds (quinones) that differentially adsorb carbon dioxide depending on their oxidative state into applications would ensure a higher CO2 adsorptive capacity than that of PSA and TSA systems.
Results
In my view, the results are adequate to arrive at the study’s conclusions. To demonstrate the efficiency of their CO2 capture system, the researchers measured quinone consumption, energy efficiency, and bed utilization through a series of experiments. Further, these adsorption characteristics were compared with those of the PSA and TSA operations to establish that ESA is a better technique. The CO2 release rate and higher adsorption cycles support the conclusions made.
Improving the Study
The research involved closed system experiments in measuring the technology’s CO2 adsorption capacity. Gas mixes (N2 and CO2) were also tested, but oxygen was not included. I would improve this experiment by using an air mixture enriched with different CO2 concentrations to test the system’s efficiency in capturing and releasing carbon dioxide. I would also include other adsorbent compounds besides quinones in the experimental tests.
Identified Items
Selection bias is lacking in this article because no human or animal subjects were sampled. The experiment involved testing a CO2 capture method based on standard laboratory protocols. Participation bias, which results from non-response, is also not applicable to this study, as no human subjects were involved. The MIT Energy Initiative Seed Fund grant sponsored the research. There is no funding bias since the researchers retained proprietary rights to their innovation and plan to commercialize it through a startup, Verdox.
The researchers tested the battery at different N2 and CO2 saturation rates to determine the optimal carbon dioxide capture conditions. They based their study on scientific premises but did not influence the results to fit a preconceived model. Therefore, there is no evidence of confirmation bias in the study. The article was peer-reviewed, as the Energy and Environmental Science journal only publishes scientifically valid findings reviewed by experts. The effect of confounding factors was minimized using N2 and CO2 saturated electrodes and measuring the adsorption and release levels. Additionally, the experiments were conducted in closed systems (controlled conditions) to eliminate confounders.
The popular article
The popular article could benefit from additional information related to experimental design and measurements. I would add material on the battery model and variables measured – CV and CO2 moles – to enlighten the reader about the study’s findings. Another important addition would be the CO2 adsorption and desorption measurement. The popular article could be strengthened or made clearer to the reader by including graphical analyses comparing the variables measured and pictures of the new battery. As a result, the public will understand its design and potential applications. Readers could better understand the carbon capture topic if the popular article included detailed information about the greenhouse effect and its relevance to the carbon cycle. Further, an explanation establishing the link between anthropogenic activities and the rising atmospheric CO2 would be useful. A review of PSA and TSA systems would expose readers to different approaches to carbon capture and the progress and promising innovations in this area.
References
Shuttle, A. (2020). A new kind of battery that removes carbon dioxide from the air?Sciworthy.
Voskian, S., & Hatton, T. A. (2019). Faradaic electro-swing reactive adsorption for CO2 capture. Energy & Environmental Science, 12, 3530-3547.