Abstract
Cytotoxicity is among the essential elements for biological assessment in vitro research. Chemicals such as drugs have unique cytotoxicity mechanisms such as cell membrane destructions and hindrance of protein synthesis. The paper aims to examine cell viability with the LDH assay on the surrounding cell media and the resazurin reduction assay evaluating cultures of live cells generating findings in HepG2 liver cells that have been treated with changing the concentration of hydrogen peroxide and cell viability measured by colorimeter assay. There is an application of concepts formulated to detect damaged or dying cells involving LDH release measurement as an indicator of membrane integrity and the caspase measurement to demonstrate cells passing through apoptosis. The release of LDH as a cell marker with a membrane compromisation and apoptosis has been shown to enhance applying similar exposure hydrogen peroxide concentration needed to induce or kill apoptosis in HepG2 cells. There is an indication of the impact of hydrogen peroxide concentration on the HepG2 cell capacity to lower resazurin. These results demonstrate the advantages of understanding the mechanism of cell death as a prerequisite for selecting an appropriate assay approach. Choosing the right strategy among the assay is critical in identifying reliable and accurate results.
Introduction
The development of drugs represents an expensive and lengthy process. It entails various phases, from identifying targets to optimize and discover final elements of drugs, clinical and pre-clinical trials involved in clinical use approval (Kocherova et al., 2020). The cell viability measurement plays a critical function for every type of cell culture. Its primary role in research is in toxicity assay, or it might be applied to cell behavior correlation to cell number (Aslantürk, 2018. The essentiality of cell viability is for screening the cell’s reactions against a chemical agent or drug. Pharmaceutical organizations mainly apply the assay to assess the impact of established cell agents. Researchers often use cell-based assays for screening compound collections to identify whether test molecules influence cell multiplication or demonstrate a direct cytotoxic effect that eventually results in cell death (Riss et al., 2016). Their use can also measure the binding of receptors and different signal deduction occurrences that might entail the expression of cellular component trafficking, organelle function monitoring, or genetic reporters. This paper examines the cell-based essay of resazurin reduction and lactate dehydrogenase (LDH) to measure viable cells.
Resazurin Reduction Assay
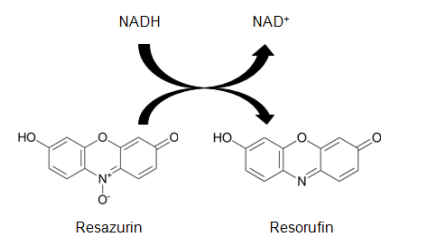
Different designs of resazurin are often becoming a joint viability agent in the use of drug screening. Resazurin is a permeable redox cell factor whose application monitors the number of viable cells with standards similar to tetrazolium compound utilization (Travnickova et al., 2019). There can be the dissolution of resazurin physiological buffers leading to a solution that is deep blue in color and direct addition to cell culture in a homogeneous form. Active metabolism of viable cells might lower resazurin into a product of resorufin, which is fluorescent and pink (Figure 1). The monitoring of the essay can be through absorbance even though there is minimum sensitivity loss. Resazurin reduction is accomplished through diaphorase-type or reductase enzyme from the cytosol and mitochondria (McGaw et al., 2014).
Conversion of Resazurim into Resorufin
LDH Assay
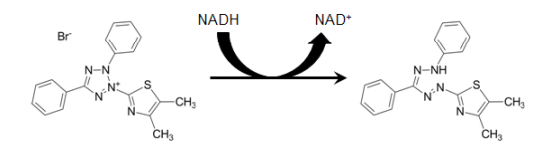
LDH assay is a colorimetric way of cellular cytotoxicity assay. The assay application uses various cell-mediated cytotoxicity assays and cytotoxicity evaluation mediated through toxic chemicals and other elements testing (Bopp et al., 2008). The assay measures the cytosolic, LDH, and stable enzyme quantitatively. The LDH is found in the cell cytoplasm. When there is a reduction of cell viability, the plasma membrane enlarges; hence there is a release of LDH enzyme into the culture medium of the cell (Kendig and Tarloff, 2007). LDH released is measured with enzymatic responses leading to conversion of tetrazolium to formazan, red through diaphorase.
Conversion of LDH into Formazan
Aim
To examine cell viability with the LDH assay on the surrounding cell media and the resazurin reduction assay evaluating cultures of live cells.
Objectives
- To establish an appreciation and understanding of the drug development process.
- To critically assess the strengths and weaknesses of a range of biochemical skills applied in evaluating pharmacological activities.
Results
In indicating a single strategy to understand the kinetics of damaged cells in the approach system, there is a comparison of resazurin reduction essay with LDH assay models to measure the impact of the concentration of hydrogen peroxide and exposure duration applying HepG2 cells. The application of concepts formulated to detect damaged or dying cells involving LDH release measurement as an indicator of membrane integrity and the caspase measurement to demonstrate cells passing through apoptosis. The multiple endpoint measurements provide data to affirm the transition timing of viable and destroyed cells between the toxin treatment time. The information demonstrates facts on the death of cells in a particular approach is vital to formulate an essay program to reduce artifacts.
LDH Release Results
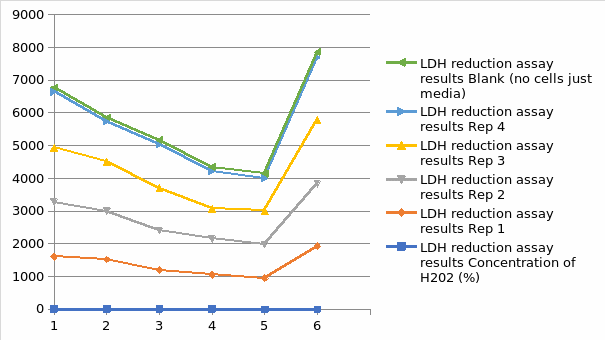
Figure 4 demonstrates the impact of hydrogen peroxide on the integrity membrane of HepG2 cells by measuring LDH release to the surrounding medium culture. The limited incubation time is the in the homogeneous standards for the fluorescent. The release of LDH benefits speed and prevents artifacts seen for limited incubation time with resazurin reduction assay. The hydrogen peroxide concentration and exposure duration were similar to that applied for the resazurin assay. A significant fluorescent signal shows evidence of destroyed cells that have produced LDH into the pathway involving this assay of cytotoxicity model. The fluorescence signal from culture medium samples after hydrogen peroxide addition was constant for every concentration of hydrogen peroxide tested. (Figure 3). The fluorescent at zero levels represents the LDH activity background present in fetal bovine serum applied to supplement the medium. After a prolonged period of exposure to hydrogen peroxide, there is an increase in the level of LDH release to the culture medium.
LDH Reduction Assay Result Graph
Resazurin Reduction
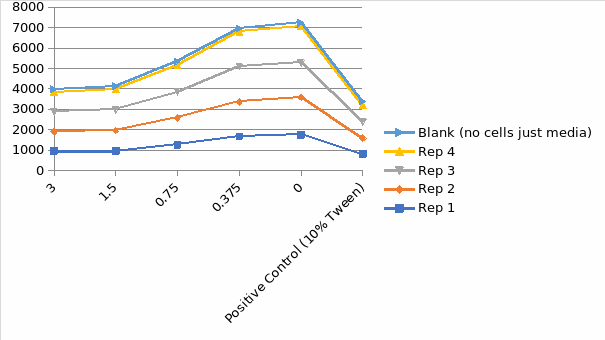
Figure 5 indicates the impact of hydrogen peroxide concentration on the HepG2 cell capacity to lower resazurin. The assay of resazurin reduction agents is redox indicators that produce a signal proportional to viable cell metabolism. There is an application of the resorufin fluorescent qualities to monitor the resazurin cellular reduction to resorufin. The reagent for the reduction needs an incubation period to enhance the viable cell population to lower the indicator dye to design a signal; hence the information at zero represents the period between hydrogen peroxide and reagent addition and involves a limited incubation time with cells needed to establish a sign. The number of hydrogen peroxide control is almost seven times the signal in a medium with dead cells or no cells. The application of metabolic indicators correlates to the number of viable cells for most general assay conditions. It is essential to realize that every one of these approaches has disadvantages when there is disruption of chemical cell metabolism leading to shifts in the signal production per cell.
Resazurin Reduction Assay Result Graph
Comparison of LDH and Resazurin Reduction Assay Result Histogram
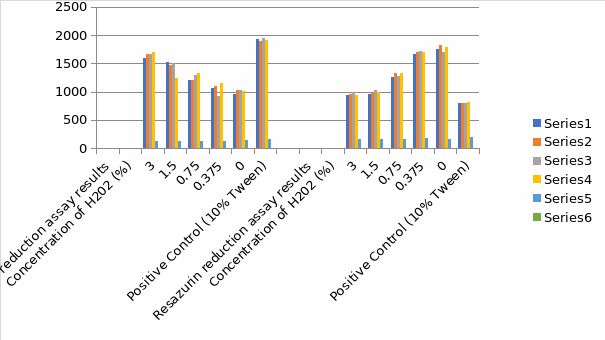
For the two assays, there was an efficient calculation of the concentration. For the exposure of the HepG2 cells liver cells to the concentration of the hydrogen peroxide, the most sensitive assay was the LDH, which indicated the highest EC value. The deviation between the two assays was not statistically important for none of the elements one form ANOVA, Turkey’s test, p is less than 0.05. There was a determination of inter-and intra-assay variability to compare the two assays. There is the calculation of coefficient variance at low and high levels. Resazurin reduction assay demonstrated low variability at significant levels and higher variability at reduced levels of cytotoxicity, while LDH indicated a higher level of intra-assay variability. There is an observation of the highest ratios of LDH at higher hydrogen peroxide concentration with a 2.2 ratio.
Discussion
The paper aimed to examine cell viability with the LDH assay on the surrounding cell media and the resazurin reduction assay evaluating cultures of live cells generating findings in HepG2 liver cells that have been treated with changing the concentration of hydrogen peroxide and cell viability measured by colorimeter assay. An in-depth characterization of the viability concept system applied for the cell-based essay can result in higher confidence in the influence of cytotoxicity screening. The data shown in this report demonstrates experimental examples of functional first cytotoxic reaction characterization of approach systems applying various cell-based assay strategies. Although the high concentration of hydrogen peroxide might not mirror the HepG2 cell concept situation, it indicates different traits of cytotoxicity measuring in cell population cultured. Figure 4 and figure 5 illustrate the general idea of the impact of toxin concentration and percentage of exposure length leading to cell death.
The data indicates that a short period of exposure to toxicity can have little or no effect on the concept system. It is also essential to understand that the range of some markers can shift with time, and hence it is vital to apply appropriate controls. The data indicates the essentiality of knowing the forces of cell destruction in the approach system being applied before selecting a period for collecting information. Various compounds in the chemical library induce cytotoxicity, which can cause distinct mechanisms; hence, focused concentration is needed to the concentration used and exposure duration when screening elements to identify toxicity. A more comprehensive level of engagement for every chemical compound will assist in guaranteeing cytotoxic characteristics detection (Tabernilla et al., 2021). The results of both assays of cytotoxicity screening ultimately rely on the cell’s responsiveness to the tested toxins.
Apoptosis marker measuring might provide additional data on the cell death mechanisms. The apoptosis timing marker assays might be vital in differentiating between necrosis and apoptosis; the apoptotic process is transient and constantly results in secondary necrosis in toxicity (Huber et al. 2016). An example is that cells that undergo apoptosis in toxicity will eventually lose integrity membrane and lead to LDH release. There is an indication of the same pattern considering the marker in HepG2 death cell in both models. The similarity is unique regarding the distinctions between the resazurin reduction and the LDH release assay. In cytotoxicity, screening uses what has considered whether the cell population is dead or viable at the end of the treatment period. Other cytotoxicity assays include ATP essay, luminometric, real-time viability, MTT, dye exclusion, trypan blue, fluorimetric, and crystal essays. The advantages of LDH are speed, simple evaluation, and high reliability. The major disadvantage of LDH assay is the serum, and other elements entail inherent LDH performance. Resazurin reduction, also known as alamarBlue, is more sensitive, cheaper, and multiplexed with different assay approaches (Aula et al., 2015). The only disadvantage is that there can be possibilities of assuming direct toxic and fluorescent disruptions from test elements impacts on the cells.
Reflection
In summary, the definition of the application of single assay to group the reactiveness of cell line in cytotoxicity assay, the one assay strategy have other uses where understanding the reaction of cells in toxicity is vital to data evaluation. Assays of cell viability are mainly applied as a regulation to confirm the outcomes of other assay approaches. For example, data cell viability can be applied to differentiate the distinctions between certain down-controls of a gene expression of a reporter and non-toxic impacts on the cells. The collection of cell viability information can be from parallel samples or, in other cases, might be evaluated by removing more pieces from the experiment. Measuring more endpoints might act as an internal regulation to affirm the outcomes of cytotoxicity assays. To guarantee maximum benefits of applying the advance homogeneous assay designs needs some selection of system concepts. Identifying the cause of dying cells in reaction to chosen control toxins will assist in understanding cell-based variables and resulting in a more crucial decision of a marker endpoint to identify. Regulating differences in the method systems by observing the protocol operating processes and measuring standard endpoints will enhance information collected from cytotoxicity assays.
Reference Lists
Aslantürk, Ö.S., 2018. In vitro cytotoxicity and cell viability assays: principles, advantages, and disadvantages (Vol. 2, p. 64). InTech.
Aula, S., Lakkireddy, S., Jamil, K., Kapley, A., Swamy, A.V.N., and Lakkireddy, H.R., 2015. Biophysical, biopharmaceutical, and toxicological significance of biomedical nanoparticles. RSC Advances, 5(59), pp.47830-47859.
Bopp, S.K. and Lettieri, T., 2008. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacology, 8(1), pp.1-11.
Huber, J.M., Amann, A., Koeck, S., Lorenz, E., Kelm, J.M., Obexer, P., Zwierzina, H. and Gamerith, G., 2016. Evaluation of assays for drug efficacy in a three-dimensional model of the lung. Journal of cancer research and clinical oncology, 142(9), pp.1955-1966.
Kendig, D.M. and Tarloff, J.B., 2007. Inactivation of lactate dehydrogenase by several chemicals: implications for in vitro toxicology studies. Toxicology in Vitro, 21(1), pp.125-132.
Kocherova, I., Kempisty, B., Hutchings, G., Moncrieff, L., Dompe, C., Janowicz, K., Petitte, J., Shibli, J.A. and Mozdziak, P., 2020. Cell-based approaches in drug development–a concise review. Medical Journal of Cell Biology, 8(1), pp.44-49.
McGaw, L.J., Elgorashi, E.E. and Eloff, J.N., 2014. Cytotoxicity of African medicinal plants against normal animal and human cells, Toxicological Survey of African Medicinal Plants. Elsevier London.
Riss, T.L., Moravec, R.A., Niles, A.L., Duellman, S., Benink, H.A., Worzella, T.J. and Minor, L., 2016. Cell viability assays. Assay Guidance Manual [Internet].
Tabernilla, A., dos Santos Rodrigues, B., Pieters, A., Caufriez, A., Leroy, K., Van Campenhout, R., Cooreman, A., Gomes, A.R., Arnesdotter, E., Gijbels, E. and Vinken, M., 2021. In Vitro Liver Toxicity Testing of Chemicals: A Pragmatic Approach. International Journal of Molecular Sciences, 22(9), p.5038.
Travnickova, E., Mikula, P., Oprsal, J., Bohacova, M., Kubac, L., Kimmer, D., Soukupova, J. and Bittner, M., 2019. Resazurin assay for assessment of antimicrobial properties of electrospun nanofiber filtration membranes. AMB Express, 9(1), pp.1-11.