- What is Chlorine?
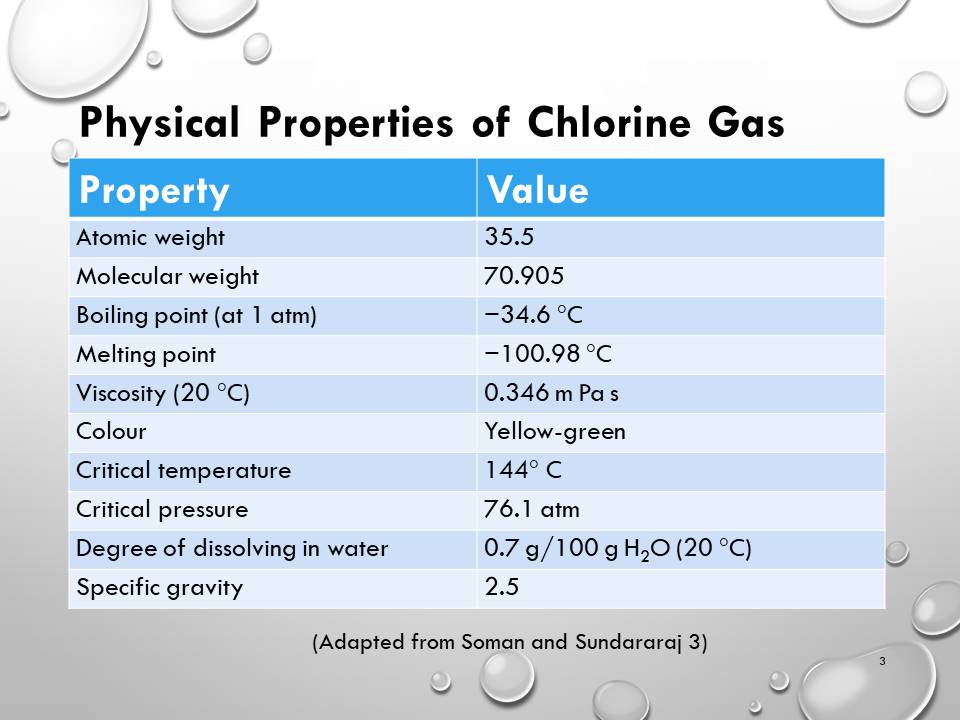
- Physical Properties of Chlorine Gas
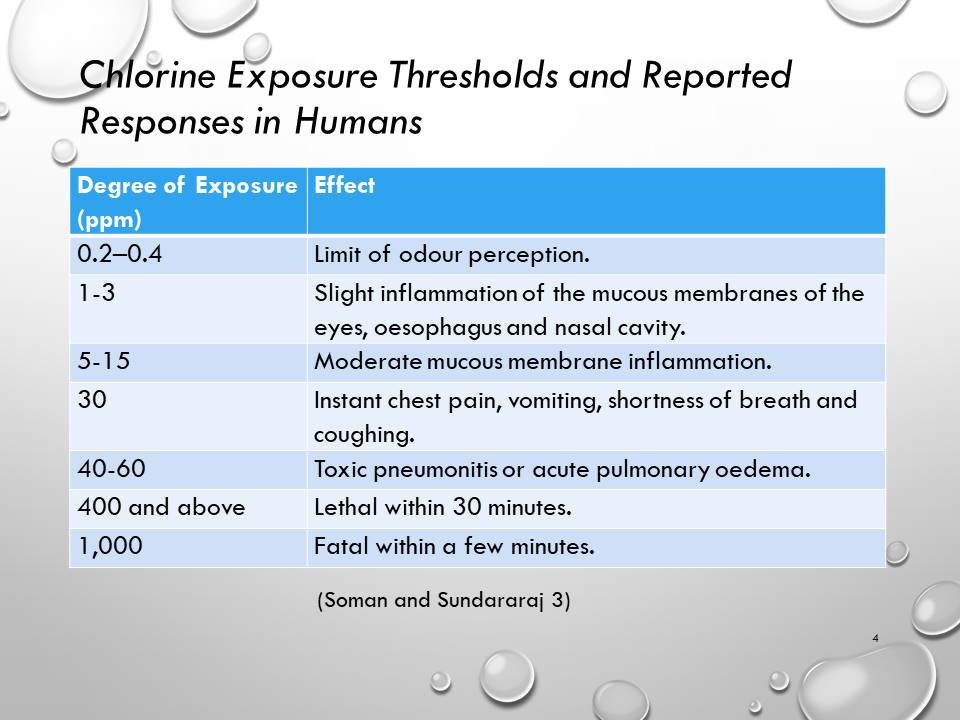
- Chlorine Exposure Thresholds and Reported Responses in Humans
- Case Study: Massive Leak of Liquefied Chlorine Gas, Henderson, Nevada (May 1991)
- Control Measures
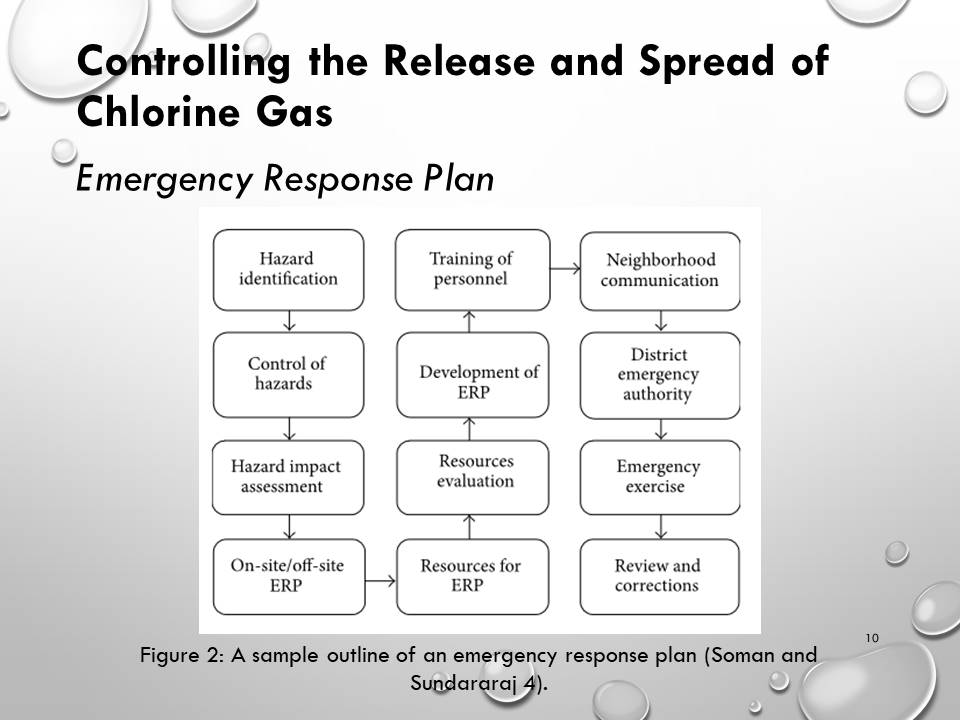
- Controlling the Release and Spread of Chlorine Gas
- Decontamination Process Following An Incident With Chlorine Gas
- Conclusion
- Works Cited
What is Chlorine?
- Chlorine is a greenish-yellow gas.
- It has a strong irritating smell.
- Chlorine is a noxious, dense gas.
- It is widely used in chemical industries.
- Gas enters the human body through the respiratory system.
- Occupational exposure threshold -1 mg/m3 (Soman and Sundararaj 1).
- The density of chlorine gas causes it to accumulate at the ground level. As a result, an unforeseen release of the gas can exceed its threshold levels and cause human injury or death.
- Chlorine gas inflames the mucous membranes found in the eyes, the lungs, air passages and spine (Soman and Sundararaj 1).
- The gas can enter into the human body through the respiratory system.
- Small quantities of chlorine can reach the threshold of acute poisoning within a very short time, thereby leading to casualties.

Physical Properties of Chlorine Gas
Chlorine Exposure Thresholds and Reported Responses in Humans
- The limit of odour perception usually differs from one person to another.
- The irritation at exposure levels of 1 to 3 ppm can be endured for approximately one hour.
- Instant chest pain, vomiting, shortness of breath and coughing.
- Other symptoms that may be present at exposure levels of 40 to 60 ppm include upper airway blockage, abdominal pain and oesophageal damage.
Case Study: Massive Leak of Liquefied Chlorine Gas, Henderson, Nevada (May 1991)
- Chlorine gas formed cloud in Henderson, Nevada (USA).
- Leak occurred on May 6, 1991 (Routley 1).
- Over 200 people received medical checks.
- 30 people remained at the hospital for treatment (Routley 1).
- 2,000 to 7,000 people evacuated (Routley 1).
- Dried chlorine + water = hydrochloric acid (corrosive).
- The corrosion created a pin-sized hole in a pipe.
- A large leakage of liquid chlorine gas formed an unsafe cloud of poisonous gas over the Henderson metropolitan, Nevada (USA).
- The incident occurred during the morning hours of May 6, 1991 (Routley 1).
- More than 200 people were checked for damage due to inhalation of the deadly gas.
- About 30 people remained at the hospital for treatment (Routley 1).
- Between 2,000 and 7,000 people were forced to vacate their homes (Routley 1).
- Smell intensified in 1 hour.
- 2 emergency calls were made.
- A command post established half a mile sloping upwards.
- Post was later relocated.
- A state of emergency was announced after 3 hours.
- Approximately 70 tons of liquid chlorine leaked.
- The smell of chlorine gas intensified within an hour and prompted two emergency calls to be made.
- Emergency responders arrived on the scene and established a command post half a mile sloping upwards.
- The post had to be relocated later because the gas had enveloped the original site.
- A state of emergency was announced following 3 hours of the first alarm signal.
- Approximately 70 tons of liquid chlorine had escaped and vapourised into the atmosphere before the leak could be stopped (Routley 2).
- Metal corrosion hampered the stoppage of leak.
- A Teflon plate stopped the leakage.
- The response team comprised firefighters and plant employees.
- The team wore level B PPE and SCBA.
- The leak stopped about 7.5 hours following the detection of the pin-sized hole.
- The gas diffused completely after an additional 3 hours.
- People returned safely.
- Efforts to prevent the leak using a blank flange were ineffective due to metal corrosion.
- A plate covered with Teflon was finally used to stop the leakage.
- The response team comprised firefighters and plant employees.
- Level B PPE provides the highest level of protection of the respiratory system. However, this gear provides a lower level of skin protection.
- This protection is used in scenarios where maximal respiratory protection is needed such as in cases involving toxic gases.
- Overall, environments with oxygen levels of 19.5% or less necessitate the use of level B PPE.
- The precise situations that require this level of protection include accessing heavily guarded radiation areas to save lives or protect valuable property.
- SCBA means self-contained breathing apparatus.



Control Measures
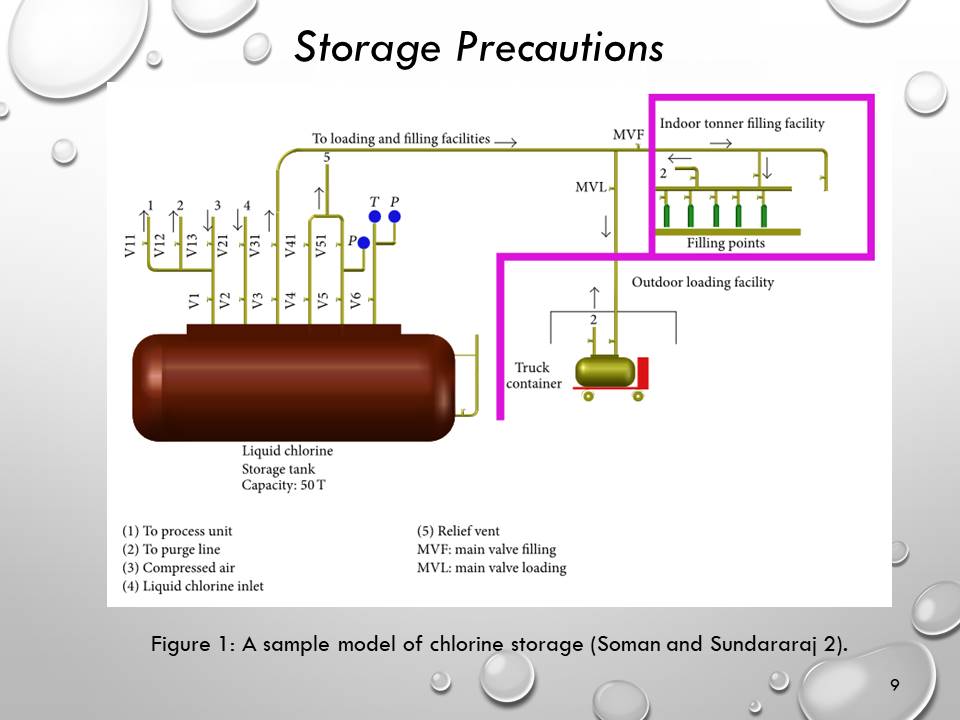
Storage Precautions
- Chlorine is liquefied and kept horizontally in cylindrical tanks.
- Safety measures of storage tanks:
- A safety vent system
- Rupture disc
- Level gauge
- Pressure metre
- Safety valve inlet
- Outlet connections
- Due to the dangers posed by exposure to chlorine, it is necessary to store it under conditions that minimise potential accidents and poisoning.
- The tank should be kept at a partial pressure of approximately 3 kg/cm2 (Soman and Sundararaj 2).
- The ideal storage temperature for chlorine is -10 °C.
- The transportation of liquefied chlorine should use compressed dry air at a density of 10 kilograms per cubic metre.
- The truck container holding chlorine gas should be kept outdoors.
- This truck should also have provisions for safe loading and emptying.

Controlling the Release and Spread of Chlorine Gas
Emergency Response Plan
- Apart from the storage precautions, emergency response plans (ERPs) strive to protect people, property, and the environment from harm in unexpected situations.
- The tasks involved in an emergency response are time-sensitive, meaning that they should be performed within the stipulated time to avoid dire consequences.
- The processes need to involve efforts from all levels of the organisation to ensure the wellbeing of workers, property and the surroundings.
- The sample response plan considers the recognition of hazards, prediction, and prevention.
- These three steps should be carried out before the development of the ERP to identify possibly dangerous events that could happen in an industry and determine the likely control measures.
The ERP should contain the following activities:
- Recognition of dangerous chemicals and associated facilities.
- Release situations and estimated rates of discharge.
- Consequences of release in terms of toxic amounts.
- Dispersion assessment for toxic release.
- Development of site strategy for damage control.
- Identification of the susceptible areas.
- A detailed ERP is required to ensure that all pertinent plants regarding the emergency operation is considered beforehand.
- Categorisation of process units with the highest probability of creating on-site and off-site emergency schemes should be part of the plan.
- Recognition of the facilities that are present in the susceptible areas is also a fundamental part of the ERP.
- The ERP should identify the prerequisites of different departments for coping with emergency situations.
Emergency Response Team
The development of an emergency response team should:
- Identify the incident controller at the site.
- Apportion resources to assuage the effects of chlorine release.
- Identify the person in charge of effective communication.
- Determine specific protective actions such as evacuation or shelter.
- Include previous reports of emergency situations.
- Include consequence evaluation reports.
- The execution of the ERP requires a team of emergency responders.
- Names of the resource person who will act as the emergency controller at the emergency control centre (ECC) should be provided.
- Before developing an ERP, all involved employees and departments should be identified and informed about their roles in the ERP.
- It is also necessary to identify all the required resources and their distribution as well as the person accountable for recovery actions.
- Finally, all members in the organization should receive training concerning the handling of the emergency effectively.
Onsite Emergency Plan
- An alarm is set off.
- The officer in charge responds accordingly.
- The ECC is activated by the officer in charge.
The ECC should have the following:
- Updated replicas of the ERP and execution procedures.
- Emergency telephone numbers.
- Details about outside agencies, response organisations and adjacent industries.
- An onsite emergency is a form of crisis that occurs in a factory and its effects are restricted to the factory grounds.
- Additionally, such crises only affect workers within the factory.
- The emission of chlorine from the storage source is usually detected by a sensor, which then sets off an alarm.
- The officer in charge during a particular shift is the ideal person to respond to the alarm first.
- The ECC is activated by the officer in charge of the operation when the crisis occurs.
- An on-site emergency plan should have a scale plan of the factory together with a list of hazardous chemicals dealt with.
- A computer with dispersion representation software.
- Material safety data sheets (MSDS) for all substances within the plant (Soman and Sundararaj 5).
- Maps of the plant and adjacent areas.
- Recent reports showing the assessment of the effects of chlorine emission.
- An emergency power supply.
- The availability of personal protective equipment.
- Where to find PPE.
- Plan maps.
- The amounts of each chemical and their locations with respect to the surrounding area should also be documented.
- A dispersion model software helps to visualise the spread of the gas, hence informing remedial measures. This computer should also have a reliable Internet connection.
- Sufficient communication amenities such as mobile phones, land telephone and fax machine to facilitate communication with internal and external agencies.
- Plan maps should indicate the following:
- Zones disturbed by the incident,
- Location and motion of vapour clouds,
- Location of the response teams,
- Evacuation areas and meeting points.
Emergency Response
The in-charge person fulfils the following roles:
- Directs the preparation of an ammonia torch and breathing gear.
- Inspects the precise location of the release.
- Scrutinises the state of the emission.
- Identifies the location of the seepage.
- Establishes actions for corrective measures.
- Informs the head of the process control room concerning the chlorine release.
- Alerts the rest of the workers.
- Checks the direction of the wind.
- Watches the surroundings.
- An ammonia torch and breathing gear are vital tolls to help identify the exact source of leakage.
- Ammonia gas reacts with chlorine gas to form ammonium chloride, which appears as a dense, white vapour.
- The in-charge person uses sounds such as sirens to alert the rest of the workers about the incident.





Decontamination Process Following An Incident With Chlorine Gas
Technical Decontamination
- Cleaning is done in three different areas.
- The exclusion zone (hot zone)
- Soiled tools are placed on a plastic piece of paper.
- Contaminated waste is discarded.
- Decontamination is described as the procedure used to eliminate or defuse a threat from the surroundings, property or living entities.
- The main goals of this process are to preclude additional harm and augment the probability of complete clinical recovery or renewal of the object endangered by the hazard.
- Two forms of decontamination are possible: technical and patient decontamination.
- Technical decontamination entails the cleaning of vehicles, apparatus and personal protective gear that have come into contact with the contaminant.
- The exclusion zone is likely to have hazardous concentrations of chlorine gas.
- Washing is done in three different areas based on the likely concentration of the contaminant.
- The contamination reduction zone (warm zone)
- Washing of primary garments such as
- boots
- outer gloves
- suit
- mask
- Removal of primary attire.
- Cleaning of secondary garments.
- Removal of face piece.
- Removal of boots.
- Removal of inner gloves.
- Moderate quantities of the contaminant can be found in the decontamination reduction zone.
- Secondary garments include inner protective clothing and inner gloves.
- Decontamination is among the most important steps to take during emergency responses.
- Decontamination, which is sometimes shortened as “Decon”, minimises the effects of dangerous substances as well as terrorist agents following the contamination of the responders or members of the public.
- Decontamination also confines the hazardous substances to specific regions.
- Three distinct zones are known: hot, warm and cold.
- The initial decontamination processes need to be restricted to the hot and warm zones to avoid cross-contamination.
- The hot zone contains the toxic agent, responders.
- Decontamination efforts begin in the warm zone, which surrounds the site of the incident. This area may also be referred to as the contamination-reduction passageway.
- The areas surrounding the warm zone are relatively safe because they do not contain hazardous materials.
Medical or Patient Decontamination
Involves three stages of decontamination:
- Gross
- Secondary
- Definitive
Gross decontamination entails:
- Evacuating the victims from the high-risk area.
- Removing the victims’ clothing.
- Rinsing the entire body from head to toe.
- The rinsing process lasts one minute.
- Patient decontamination is usually done when the contaminant presents additional risk to the victim or a secondary danger to the response personnel.
- Medical decontamination also necessary to avoid contaminating medical facilities with toxic substances.
- Contaminated medical facilities pose significant health risks to other patients and healthcare personnel.
Medical Decontamination
Secondary decontamination involves:
- Rinsing the entire body using clean water.
- Washing with a cleansing agent all over the body.
- Rinsing the entire body with clean water.
Definitive decontamination entails:
- Performing a meticulous head-to-toe wash until clean.
- Bathing thoroughly with large amounts of water.
- Towel drying the body and wearing clean clothes.




Conclusion
- Chlorine is a hazardous gas.
- Inhaling chlorine can kill within a short time.
- Chlorine gas should be stored to minimise the likelihood of leakage.
- Plants handling hazardous gases such as chlorine should establish safety measures.
- Workers should receive adequate training on emergency responses to gas leaks.

Works Cited
Web.Routley, J. Gordon. “Massive Leak of Liquified Chlorine Gas Henderson, Nevada.” Federal Emergency Management Agency, 1991,
Soman, Ambalathumpara Raman, and Gopalswamy Sundararaj. “Accidental Release of Chlorine from a Storage Facility and an On-site Emergency Mock Drill: A Case Study.” The Scientific World Journal, vol. 2015, 2015, pp. 1- 11.