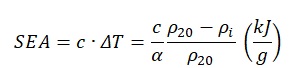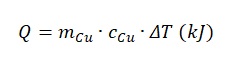For the chemical and metallurgical industry, a thorough study of the properties of the materials used and the application of reference data for analysis and possible prediction of the results of a practical application is essential. Thus, in fact, information about the material, its chemical composition and physical and chemical properties is crucial for making decisions about its usefulness in specific practical tasks. Generally speaking, the possibility of application of structural material in engineering sciences is determined by its characteristics. This approach is conditioned both by the necessity of conformity between material parameters and industrial application and minimization of damage combined with the creation of higher quality products.
The research question of this work has been reduced to the analysis of two inactive metals — silver and copper — in terms of their physical and chemical parameters to assess their potential use in industry. Characteristics of interest, and therefore to be measured in this work, include temperature, thermodynamic and physical parameters. In particular, for copper and silver, melting and boiling temperatures have been described along with the density of materials and their specific heat capacity. Electrical resistance, in combination with the coefficient of thermal stability, has also been given. In addition, given that both metals are a classic conductor of electric current, increased interest had a regression analysis of electrical resistance as a function of temperature: if both materials linearly increase the resistance, it is important to determine a more suitable material for production tasks at a given temperature. At the same time, the study of specific absorbed energy is a task for the design of reduced mass elements. It is known that this characteristic is defined as the amount of energy — whether thermal or electrical — absorbed per unit mass of the substance. Thus, at different values of the parameter, the material that absorbs more energy in electrochemical and engineering industries will have a priority advantage. For this reason, the evaluation of this parameter is an intriguing part of the study, which allows determining the effectiveness of copper and silver as structural materials.
The relevance of this topic was dictated by the need for qualitative and quantitative assessment of the two metals for the implementation of design tasks. Researches of a wide spectrum of digital scientific articles have led to the conclusion that though the comparison of silver and copper is not an innovative idea of the given work, the majority of works are focused on a narrow set direction, ignoring the general information (Hlina et al., 2017; Barahona et al., 2016). For this reason, this thesis will be the suitable material for generalizing the available information and demonstrating the logic of calculation and interpretation of some of the most important values.
Determination of Physical Properties
In order to facilitate visualization and to create a more understandable comparison, the physical properties of two metals are shown in Table 1.
Table 1. Summary information on physical properties of materials (Helmenstine, 2019; Hampson & Hanssen, 2019; Sebastian et al., 2017).
As can be seen, the data for each metal is a set of numbers, the value of which, however, is mostly more for copper than for silver. Thus, the physical properties of copper and silver are different for each of the parameters, which justify such a different use of materials in the industry. Before demonstrating the practical use of the data, it is necessary to clarify what parameters were proposed for discussion. The melting points of metals are called the temperature at which a solid crystal substance enters the liquid phase, and vice versa. The boiling point is considered to be the temperature threshold at which a liquid under constant pressure boils. At the same time, it is interesting to note that melting and boiling temperatures, although they are similar characteristics, in fact, represent completely different structural changes in materials. During melting, the liquid and solid phases are in equilibrium, whereas during boiling, the liquid begins to dominate. Density is a scalar physical quantity that depends on the structure of a crystal lattice, which is determined for a homogeneous substance by the mass of its unit volume. Thus, of two materials of the same volume, the one with the higher density has the greater mass. The specific heat capacity characterizes the amount of heat energy that must be transferred to the material in order to change its temperature by one degree. From the name of this term, electrical resistance, it becomes clear that it describes the potential for conducting an electric current: the higher the resistance, the weaker the current is conducted. Finally, the dependence of the electrical resistance of a substance on its current temperature is characterized by the temperature coefficient of resistance.
Focusing only on the numbers from the table, it is needed to make several important comments at once. First of all, it would be correct to note that copper is a «harder» metal than silver. Although silver, in fact, has a greater molecular weight and density, it is relatively good at conducting the electric current and requires less external effort to be used as a conductor. This may also be due to a large number of free electrons. Second, the situation seems to be opposite for copper: the metal has a higher boiling point and melting point, which indicates the relative complexity of its atomic structure compared to silver. Third, thermoelectric properties of copper also show a higher stiffness, namely that the metal needs more thermal energy to be heated by 1 degree: the thermal capacity of copper is higher. The higher heat capacity compared to silver makes copper more resistant to electricity transmission, and the superior electrical resistivity only confirms this fact.
In order to plot the electrical resistance dependence on the temperature, it is necessary to refer to the formula of this parameter, presented below. With the help of MS Excel software, resistance calculations for seventeen test points have been performed, and the graph shown in Figure 1 has been drawn. It is not difficult to note that the electrical resistance of each of the metals increases linearly with increasing temperature. In fact, this means that the higher the temperature of the medium, the more complex the material will function as a conductor. For example, if one had to choose which material to use for high-temperature work under high voltage, then, if all other conditions being equal, silver would be a more advantageous option.
pi = p20 [1 + α(t – 20°C)]
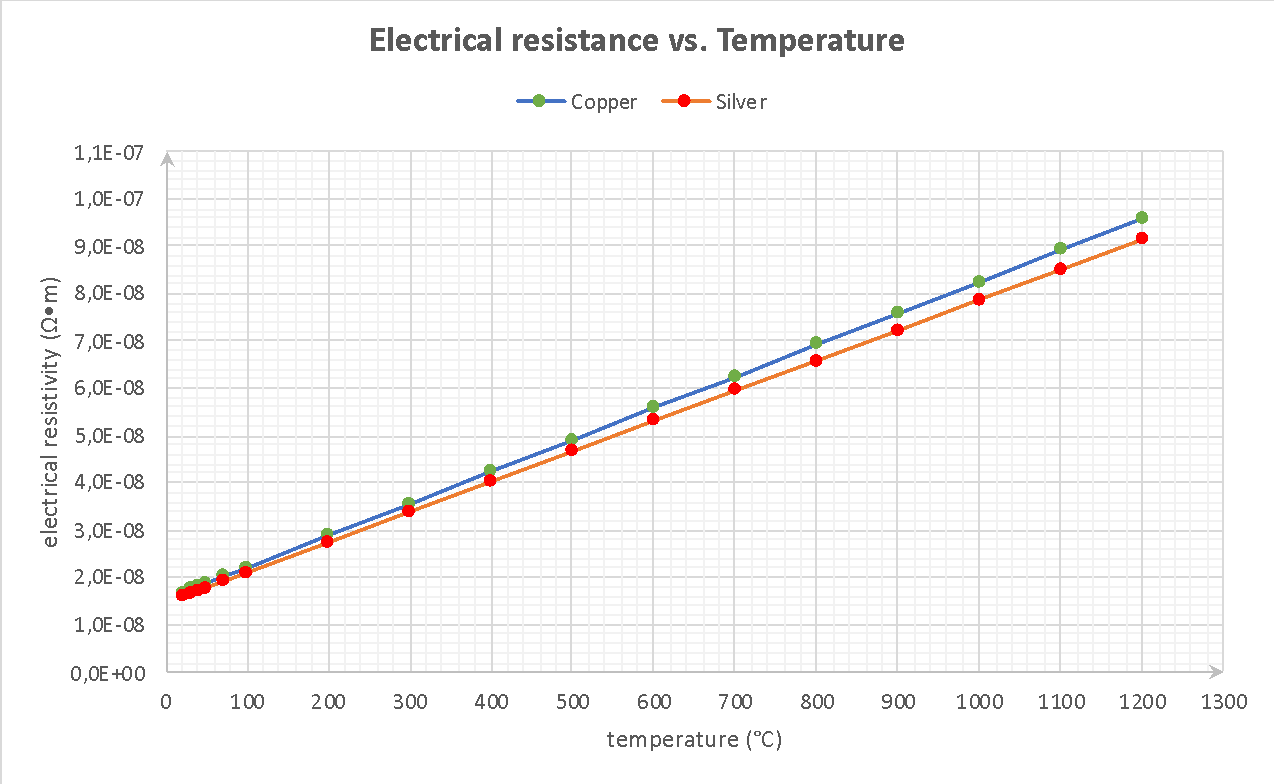
It is interesting to note that on the basis of the phenomenon of linearity of increasing heat capacity at increasing temperature is a quantum change in configurations of atoms. In particular, each of the elements has a certain reserve of vibrational and rotational levels of internal energy, which begin to excite in the case of the substance of additional heat. Since the atom heats up, their kinetic energy increases and this leads to a more active fluctuation of the atoms. Heating is accompanied by an increase in occupancy at these levels, and therefore the amount of stored energy of the metal increases.
In the meantime, the data presented above allow predicting and assessing the nature of physical properties. In particular, using the graph allows predicting the values of heat capacity of metals at specific temperatures. For instance, if in the construction industry there was a choice between using copper and silver as a material for a high voltage wire, and it would be clarified that the operating temperature of the process would reach 1300 degrees, then thanks to the graphics, it could be understood that silver would be a more convenient option.
It must be recognized that the specific amount of absorbed energy is the amount of energy (i.e., heat) that has been transferred to the substance to change its resistance. Taking into account the linearity of resistivity growth and the physical meaning of the value, the following relation can be used to establish a connection between electrical resistivity and absorbed energy:
At the same time, the amount of specific energy absorbed can be found by formula:
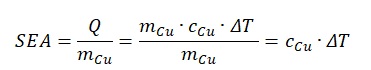
In this formula no. 4, one can see that there is a direct correlation between the specific heat capacity of the substance and the specific absorbed energy. At the same time, the value of energy is determined by the temperature difference: the more substantial the material was heated, the more energy it was able to absorb. The temperature difference is also important in the electrical resistance search formula. Thus, this temperature difference (ΔT) in the formula no. 4 is the equivalent of that used in the electrical resistivity formula no. 1. After expressing the temperature difference, the following equation will be obtained:
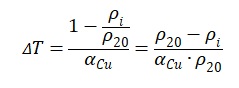
Next, if the result obtained in the fifth formula is substituted to the formula no. 4, one can get the finished expression. Moreover, it should be remarked that instead of generalized values of specific heat capacity, electrical resistance and temperature coefficient of resistance is more correct to use the known values.
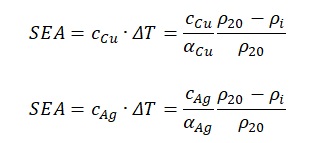
In general, it is not difficult to notice several regularities that can be detected when addressing the formula no.2. The first point to pay attention to is the direct dependence of unique absorbed energy on the heat capacity of the material: the higher one of these indicators, the higher the other. In this case, one should pay attention to the denominator, which contains a temperature coefficient of resistance. If one substitutes the known values for silver and copper in formulas no. 6 and no. 7, then the following expressions should be obtained:
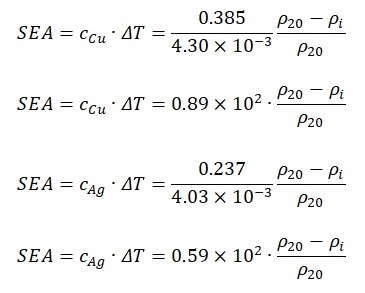
Based on formulas no. 9 and no. 11, it is needed to be said that the first term of the equation for silver SEA is comparable to the same value for copper. By substituting the remaining value p20 in the formulas, one will obtain:
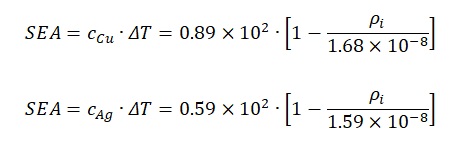
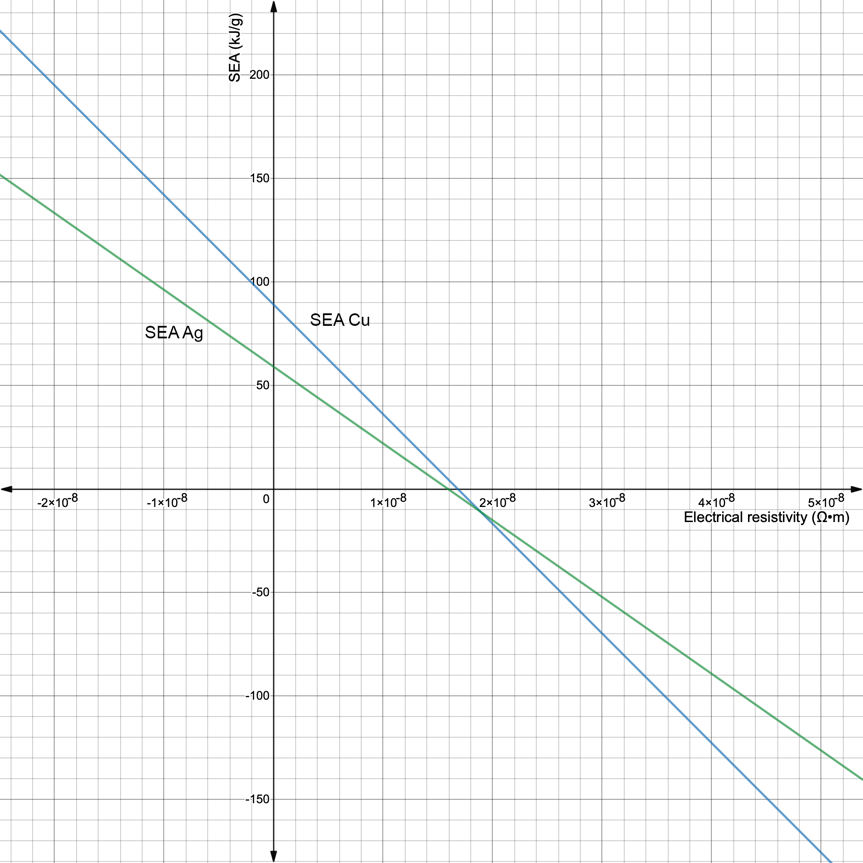
The final step to compare the two elements using the above formulas will be to determine the nature of the dependence of specific absorbed energy on the type of metal. Taking into account that in formulas no. 12 and no. 13 the only variable is the value of electrical resistance at a particular point, it is possible to plot two functions and estimate the apparent difference. As shown in Figure 2, the values of the specific absorbed energy for silver will always be higher than the SEA values for copper at the same electrical resistivity values. This result can be interpreted as follows: the dominance of the energy density of silver indicates a higher value of this material than copper.
The selection of a particular material as a source for the work should be accompanied by an analysis of its physical properties. Specific comparison criteria may include density, thermoelectric and mechanical characteristics. For instance, copper is a higher priority in the context of thermal stability and density but loses out in specific heat capacity and resistance. Silver shows better results in conducting an electric current but is itself a denser substance, which increases the weight of the product by 15% compared to copper. In sum, it is important to conclude that the choice of a particular source should be based on a sum of factors, including needs, costs, and justifiability of use.
References
Hampson, J., & Hanssen, S. (2019). Electrotechnology practice. Cengage AU.
Helmenstine, A. M. (2019). Copper facts: Chemical and physical properties. ThoughtCo. Web.
Helmenstine, A. M. (2019). Silver facts (atomic number 47 and element symbol Ag). ThoughtCo. Web.
Helmenstine, A. M. (2019). Table of electrical resistivity and conductivity. ThoughtCo. Web.
Sebastian, M. T. et al. (2017). Microwave materials and applications. John Wiley & Sons.
Hlina, J., Reboun, J., Hirman, M., & Hamacek, A. (2017).Comparison of copper and silver thick film on alumina substrates properties [PDF document]. Web.
Barahona, M., Betancourt, D., & Ellinger, F. (2016). Comparison of UWB chipless tags on flexible substrates fabricated using either aluminum, copper or silver [PDF document]. Web.