Introduction
Cells contain subcellular structures, organelles, which are suspended in the cytoplasmic fluid and play various critical roles. Animal cell organelles include nuclei, mitochondria, endoplasmic reticular, cell membrane, Golgi complex, ribosomes, microtubules, microbodies, etc. These are usually separated and purified for further analysis using biochemical techniques such as differential and density gradient centrifugation. Organelles may lead to a disease state in various ways.
Firstly, the organelle itself may malfunction because of possessing defective building blocks, which impair its role or may be damaged due to exposure to harmful agents such as heavy metals, radiations, or reactive oxygen radicals (Fitzpatrick and Jonathan 48). Secondly, the ordinary activities of an organelle can aggravate destruction occurring elsewhere in the body. In this paper, the author discusses various cell organelles, their functions, and some of the health abnormalities associated with their dysfunction.
The Nucleus
In many animal cells, the nucleus is often centrally located in the cytoplasm. It is enclosed by a double lipid bilayer, the nuclear envelope, which has pores that allow the passage of molecules of different sizes (Mclennan et al. 5). The eukaryotic nucleus carries the hereditary information of the cell contained in chromosomal structures. DNA replication and RNA transcription take place in this organelle. Also, it contains the nucleolus where rRNA is processed, and ribosomes are partially assembled (Mclennan et al. 5). The processed RNA passes into the cytosol, where protein synthesis occurs. Thus, the nucleus is the cell’s control room.
Nearly all genetic disorders are caused by mutations that occur in genes housed by it. For instance, several diseases caused by flaws in the nuclear membrane occur in genes that code for emerin and lamin. Emerin is an inner membrane protein, while lamin forms the cytoskeleton portion of the nuclear lamina (Fitzpatrick and Jonathan 50). These defects in the membrane components result in cellular abnormalities such as a fragile nuclear envelope, non-coordinated gene replication and transcription, and reduced tolerance to physical stress (Fitzpatrick and Jonathan 50). Progeria, Emery-Dreifuss muscular dystrophy, and some brain and bone defects are notable diseases in this category (Fitzpatrick and Jonathan 50).
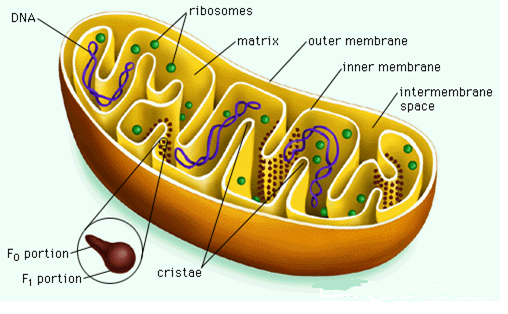
Mitochondria
These are sausage-shaped organelles, 1-2 µm in diameter, that maybe 1000-2000 in number per cell (Mclennan et al. 5). They possess a unit membrane, the outer being smooth, and the inner having protrusions called cristae. Mitochondria contain a small circular DNA molecule, mitochondrial RNA, and ribosomes for the synthesis of some of its proteins (Mclennan et al. 5). These organelles are sites for cellular respiration that generates ATP. Mitochondrial impairment can result in a myriad of deleterious events since the damaged organelle produces reactive oxygen species and triggers adverse reactions, including protein carbonylation, lipid peroxidation, DNA damage, and apoptosis by releasing cytochrome c (Damme et al. 349).
Endoplasmic Reticulum (ER)
This organelle is a system of interconnected membranes within the cytoplasm and is continuous with the nuclear envelope. The smooth ER harbors many membrane-bound enzymes that participate in lipid biosynthesis and biotransformation reactions – oxidation and detoxification of xenobiotic substances such as drugs, toxins, and radical species (Mclennan et al. 5). The rough ER has many ribosomes on its surface. The ribosomes synthesize proteins meant for secretion by the cell, or those predestined for cell membrane or specific organelles. The lumen of the rough ER specializes in post-translational modification, e.g., glycosylation, ubiquitination, sumoylation of the synthesized proteins (Mclennan et al. 5). The proteins and lipids made on the RER are ferried in specialized transport vesicles to the Golgi complex, a stack of flat membrane bags, which further modify, arrange, and target them to their final destinations (Mclennan et al. 5).
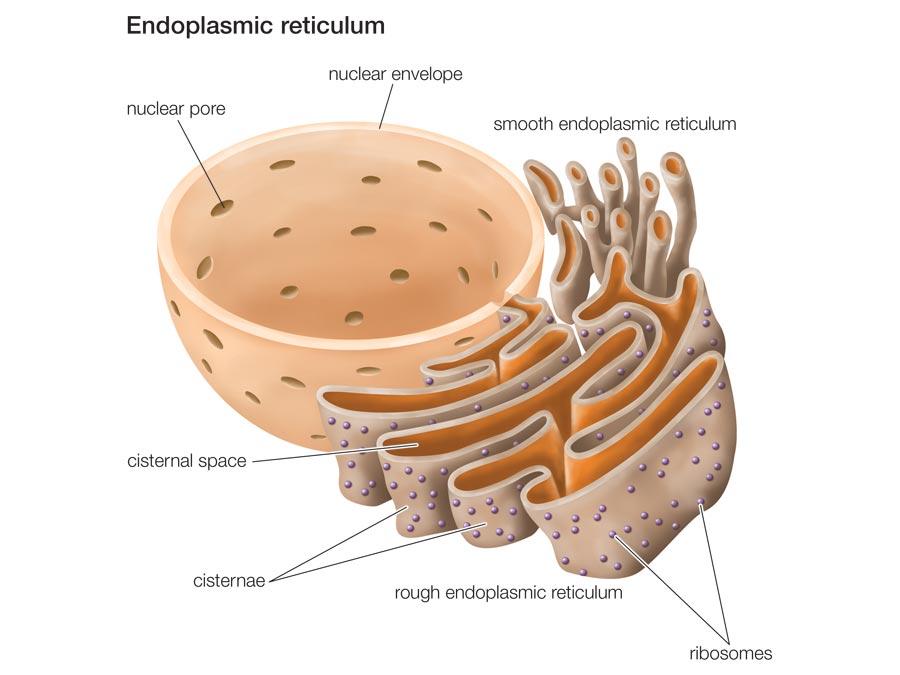
Since the ER plays very critical roles in the cell, any disruption of its function can have severe outcomes. Wrongly folded proteins transcribed from mutated genes, and those caused by ER stress are liable for many diseases, cystic fibrosis (CF) being the most conspicuous. Cystic fibrosis is a deadly heritable disease characterized by the absence of cystic fibrosis transmembrane regulator (CFTR), a type of cell membrane chloride channel (Fitzpatrick and Jonathan 53). This situation causes the deposition of thick mucus on vital organs such as the lungs and pancreas. The misfolded CFTR protein fails to leave the ER and is degraded by autophagic mechanisms (Fitzpatrick and Jonathan 54).
Mitochondria-associated membrane (MAM), a specialized sub-group of the ER has specific lipid and protein composition and is involved in cross-communication with mitochondria (Liu and Zhu 1). It is linked to the mitochondria through the mitochondria-ER tethers. It functions in phospholipid synthesis and exchange, calcium signaling, regulation of mitochondrial fission, and apoptosis (Liu and Zhu 1). Research evidence implicates the abnormalities and dysfunction of MAMs in the etiology of various neurodegenerative disorders, including amyotrophic lateral sclerosis, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, as well as diabetes and heart disease (Liu and Zhu 1). For example, upregulated MAM function and heightened ER-mitochondria communication have been linked to the pathogenesis of AD. Similarly, mutations in genes coding various MAM proteins have been associated with familial ALS and PD (Liu and Zhu 2).
Microbodies/Golgi Complex
Microbodies comprise lysosomes and peroxisomes. Lysosomes are small membrane-enclosed bodies that pinch off from the Golgi complex and possess a variety of lytic enzymes for proteins, lipids, carbohydrates, and nucleic acid breakdown (Mclennan et al. 6). Lysosomes are recycling centers for biomacromolecules from within or outside the cell. Lysosomal activities (autophagy) are vital for existence and homeostasis of terminally differentiated cells since such cells are unable to dilute accumulating harmful metabolites and damaged organelles by cell division (Damme et al. 350).
Autophagy and chaperone-mediated autophagy have also been associated with neurodegenerative disorders, i.e., frontotemporal dementia, prion diseases, and lysosomal storage diseases (Damme et al. 350). These disorders are a consequence of pathogenic mutations in several autophagy-related genes, genetic modifications that lead to either gain or loss of function of their transcription products. For instance, lysosomal storage diseases are because of the absence of some lysosomal enzymes. Their salient feature is the piling of undigested substances that cause irreversible cell damage. These disorders include Pompe’s disease, I-cell disease, Gaucher’s and Tay-Sachs disease type II (Damme et al. 350).
Peroxisomes contain the enzyme catalase. It is the site for hazardous metabolic reactions that yield highly reactive free radicals and hydrogen peroxides. Catalase hydrolyzes toxic hydrogen peroxide to generate water and oxygen. Dysfunctional peroxisomes result in the buildup of long-chain fatty acids throughout body tissues, a disorder called adrenoleukodystrophy (Damme et al. 350). This disease leads to impaired brain function, muscle stiffness, ataxia, malaise, and paralysis of lower limbs.
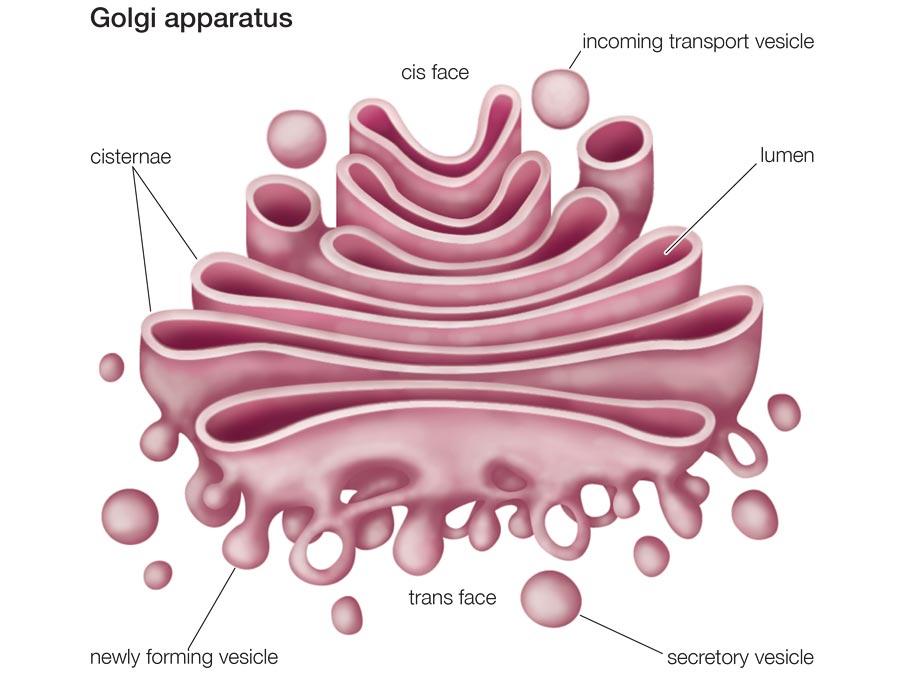
The Golgi bodies showing the fusion of transport vesicles at the cis end and the pinching of secretory vesicles at the Trans face (Petruzzello). Defects in this organelle result in a group of lethal congenital disorders of glycosylation that arise from mutations of genes for glycosylation enzymes and transport proteins.
Conclusion
Organelles play pivotal roles in a cell. Cumulatively, their activities enable the body to perform all functions of life. Malfunctions of cell organelles result in numerous diseases. The majority of these disorders are familial and hereditary thus associating mutations to their pathogenesis.
Works Cited
Damme, Markus, et al. “Autophagy in Neuronal Cells: General Principles and Physiological and Pathological Functions.” Acta Neuropathol, vol. 129, no. 1, 2015, pp. 337–362.
Fitzpatrick, David R., and Jonathan R. Seckl. “Molecular and Genetic Factors in Disease.” Davidson’s Principles and Practice of Medicine, edited by Nicki R. Colledge et al., Elsevier Limited, 2014, pp. 47-67.
Liu, Yi, and Xiongwei Zhu. “Endoplasmic Reticulum-Mitochondria Tethering in Neurodegenerative Diseases.” Translational Neurodegeneration, vol. 6, no. 21, 2017, pp. 1-8.
Mclennan, Alexander, et al. Bios Instant Notes: Molecular Biology. 4th ed., Taylor & Francis, 2013.
Petruzzello, Melissa. “6 Cell Organelles”. Encyclopaedia Britannica. Web.