Each substance is characterized by unique density values, which are determined by the atomic and molecular structure of objects. It should be admitted that the density is traditionally defined as the mass of the sample under study divided by the volume it occupies in space. Nevertheless, the phenomenon of density is characteristic not only for solids — liquids, gaseous substances, and vapors also have their densities. For this reason, if a mixture of substances presented in different aggregate states is created, it will be divided into fractions.
This situation is typical for flasks in which corn syrup, colored water, vegetable oil, pieces of aluminum foil, steel nut, and peppercorn are mixed. During the experiment, it is not difficult to notice that the mixture was divided into layers, as shown in Figure 1. This is because all components have different densities: those with higher values are located in the lower layers.
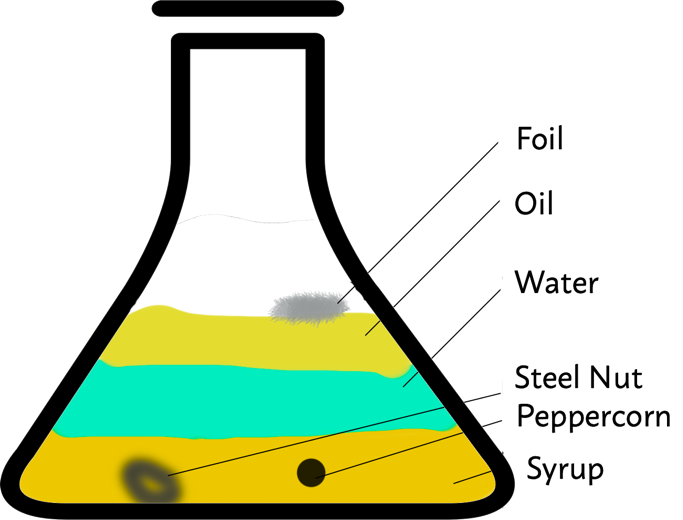
Solid components find themselves on different layers due to the buoyancy force. This force represents the weight of the displaced liquid, according to Archimedes’ principle. At the same time, the location in the mixture depends directly on the mass of the solid — the more substantial the object, the lower it drops. Thus, a black peppercorn can “freeze” in a particular area, because a balance is achieved between the buoyancy force and the weight of the pepper. The steel nut is extremely heavy, more massive than all liquid layers, so it sinks to the lowest point. Finally, the aluminum foil was on the water surface because the surface density of the material is lower than the water density: as a result, the buoyancy force is higher than the foil weight.
Water and air have a molecular structure — these matters consist of tiny elements that continuously move in space called molecules. As a rule, the fluctuations of molecules are not subject to known regularities and are generally chaotic, although their intensity can be controlled by a person. If it is necessary to make molecules of objects move faster, it is possible to transfer to them a part of external energy — for example, heat. On the contrary, to slow down the chaotic motion, it is possible to take energy from molecules by cooling the environment.
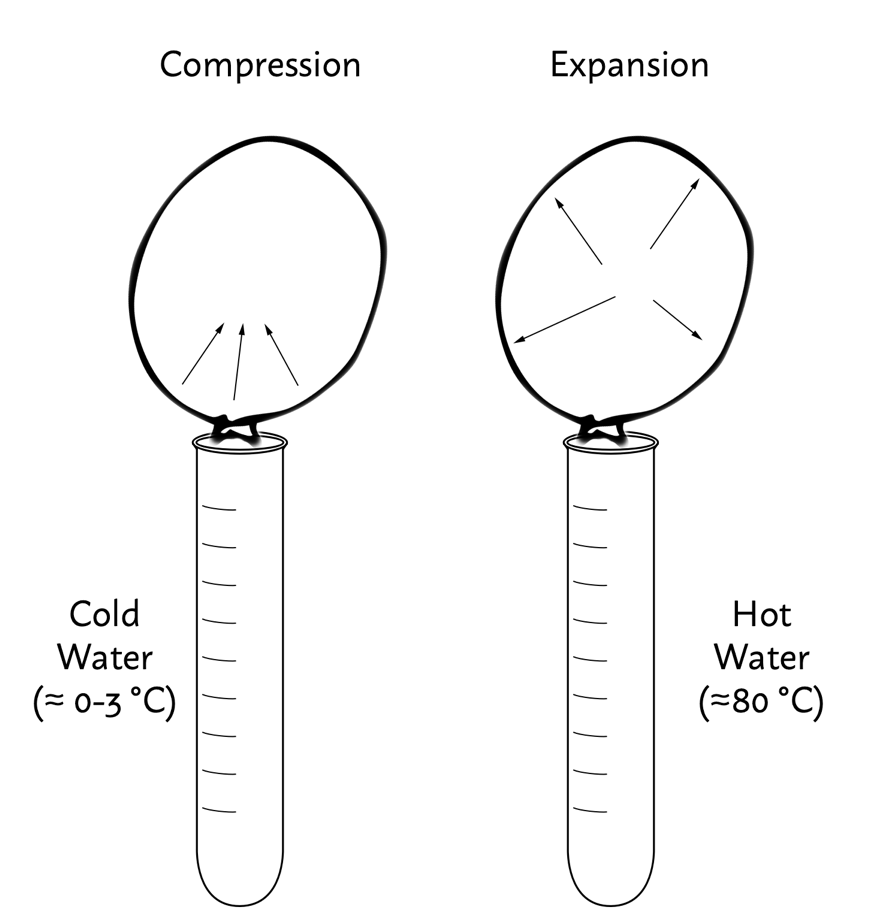
In a laboratory experiment, when a blown ball is over ice water, the kinetic energy of the molecules’ fluctuations is transferred to the water. According to the law of thermodynamics, heat is transferred from warm bodies to less warm ones: in this case, the air of the ball was hotter, so the energy was transferred to the ice water. That means that the molecules were more limited on movement, and the ball was compressed, as shown in Figure 2. In another experiment, when the balloon was placed over hot water, a similar situation occurred. This time the water was warmer, so it transferred some energy to the air molecules. As a result, the molecules began moving more intensively, which caused the expansion of the balloon.
When mixed with liquids, some substances are dissolved and converted to a liquid state. Dissolution seems to be a strictly physical process as there is no change in the chemical nature of the substance. For example, sucrose, once dissolved in water, has the same structural formula as before mixing. However, dissolution can be considered a chemical process because some compounds undergo oxidation, exchange, or decomposition reactions when immersed in water. This is typical for hydrolysis, where the submerged substance interacts with water to create new products.
Powder soda is mixed with water and actively dissolved. If vinegar is added to the dissolved soda, there will be a reaction of ionic exchange during which gaseous substance — carbon dioxide is formed. Empirically, it is observed as the formation of numerous bubbles on the surface of the solution. Other reaction products include salt and water, as shown in the equation:
It is important to clarify the answer: table vinegar is an essence in which water and acetic acid (CH3COOH) are mixed. When adding dry baking soda to the vinegar, the same gas formation reaction will be observed since soda (sodium hydrocarbonate) initially interacts with water, resulting in powder dissolution. After that, the ionic exchange between the two compounds begins, resulting in the formation of reaction products. Therefore, the interaction of soda and vinegar is both dissolution and chemical transformation.
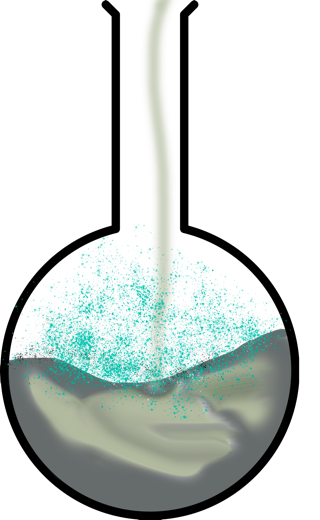
Table 1. Periodical table for food
Taking into account the Table 1, it should be kept in mind that the indicated foodstuffs can only be approximately correlated in terms of organoleptic properties. Thus, cherry and redcurrant are sour and have a red color, however, cherry is much darker. In fact, each group includes food or drinks that are only about the same in characteristics. Nevertheless, this is enough to distinguish some foods from others. For example, the characteristics “dark” and “sweet” are not exactly suited to describe an apple or lemon, but correctly describe a Coca-Cola.
The table divides into taste characteristics and color: thus, each of 28 products is located at the intersection of color and taste — for example, Liquorice can be found at the intersection of the “Bitter” column and the “Dark” line. In general, such a matrix approach well organizes and structures the variety of products, but, as noted above, does not have perfect execution.
The drawback of this table also includes the difficulty of determining the place for a large number of positions. The question is where, for example, cucumber, pork, water, mint gum, or tea should be placed. These products have a range of flavors and more sophisticated colors, such as some of those already in the table. Simultaneously, multi-colored products, such as watermelon or confectionery, cannot be assigned to specific lines.
Such a table is well-suited for a minor study of food, but it is absolutely useless from the point of view of chemistry. The periodic table, which was developed by Dmitry Mendeleev, sorts out elements, but not compounds. It is challenging to organize a perfect table for food and drinks, as these are complex substances with a variety of characteristics. On the contrary, the elements in the periodic table of elements are the fundamental blocks that all matter. For food, one can delve deeper and disassemble it into an atomic structure: for example, Coca-Cola contains orthophosphoric acid, which includes phosphorous, hydrogen, and oxygen. However, the same elements are present in fish: due to the ambiguity, it is impossible to draw up a periodic table for food.