Introduction
Fruits such as mangoes, pineapples, berries and papaya have diversified tastes and flavours and make excellent sources of various minerals and vitamins in the diet (Rababah, Ereifej, & Howard 2005, p. 4445). Among the numerous antioxidants found in fruits, vitamin C has the most significant biological role in the human body. Vitamin C helps to decrease levels of C-reactive protein (CRP) and to fight inflammations. In addition, vitamin C is a predictor of heart disease, threat of arteriosclerosis, as well as, various forms of cancer. L-Ascorbic acid is both an anti-oxidant and free radical scavenger. It is found in various citrus fruits and leafy vegetables (Brevard, Marques, Renfroe, Lee, & Gloeckner 2010, p. 760).
Vitamin C is necessary for human growth and repair of tissues, tendons, ligaments and blood vessels through the formation of collagen. The ease with which vitamin C oxidizes causes it to diminish faster in fresh fruits than in processed ones, when cooked or stored in the open air (Vasco C, Ruales J, & A 2008, p. 817). Ascorbic acid is freely soluble in water, which means tinned fruits retain their vitamin C in the liquid. Vitamin C is slightly acidic and is partly soluble in alcohol, and insoluble in Chloroform, ether and benzene (Rickman, Barrett, & Bruhn 2007). The formula for ascorbic acid is C6H8O6. Its structural formula is shown in figure 1 below:
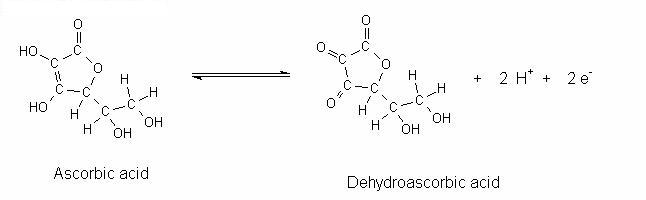
Lack of vitamin C causes Scurvy, which is characterized by weakness and small hemorrhages all over the body that cause the skin and gums to bleed, as well as loosening of teeth. Since human beings cannot store vitamin C in the body, it should be replenished on a daily basis, with a minimum requirement of 30 mg (Connell, Zoellner, Yadrick, Chekuri, Crook, & Bogle 2011).
Over 80% of vitamin C in the human diet is obtained from fruits and vegetables. However, fruits face rapid deterioration after cropping due to their high moisture content. As a result, fruits undergo various processing activities in order to reduce their moisture content, which also reduces microbiological activity. Reducing water activity improves the stability of fruits by reducing both physical and chemical reactions that occur during storage (Miller & Knudson 2012, p. 14).
Research suggests that heat treatment of fresh fruits during processing causes a significant loss on vitamin C; however, canning preserves the remaining nutrients level over a long time relative to fresh produce (Rickman, Barrett and Bruhn 2007, p. 936). The amount of vitamin C in fresh products begins to reduce immediately after harvest. Research suggests that the level of vitamin C loss depends on crop variety, as well as, grower processes that have a direct impact on vitamin C content (Koyuncy & Dilmacunal 2010, p. 96).
A variety of techniques can be used to determine vitamin C in fruits (Koyuncy & Dilmacunal 2010, p. 97). This experiment employs the new calorimetric technique that uses Folin phenol reagent to compare vitamin C content in fresh and processed pineapples and strawberries.
Project Aims and Objectives
Aims
- To determine the vitamin C (ascorbic acid) content of fresh, frozen and tinned (canned) pineapples and strawberries using new calorimetric technique that uses Folin phenol reagent.
- To compare vitamin C content in fresh, frozen and canned pineapples and strawberries.
Objectives
- To measure vitamin C content for fresh, frozen and tinned pineapples and strawberries
- To compare vitamin C content of fresh fruits (pineapples and strawberries) with literature values of vitamin C.
- To evaluate the influence of processing (freezing) and thermal processing (canning) on vitamin C retention for pineapples and strawberries
Experimental Design
This experiment will be comparing the content of vitamin C in fresh, frozen and canned fruits (pineapples and strawberries) using the direct calorimetric technique. The direct colorimetric method of is based on the assessment of the level to which a 2,6-dichlorophenol-indophenol solution is decolorized by ascorbic acid in sample extracts and in standard ascorbic acid solutions. The dye is slowly reduced by various interfering substances.
Equipment
- Refrigerator
- Test tubes
- Paper filters
Reagents
- 2% Metaphosphoric acid
- Distilled water
- Dye solution: dissolve 100 mg of 2,6-dichlorophenol-indophenol dye in hot distilled water at around 85 – 95oC. Add 844 mg of sodium bicarbonate to the mixture. After they have dissolved, cool it and obtain a 100 ml solution. Filter and dilute 25 ml with 500 ml of distilled water.
- Standard ascorbic acid solution: use 100 mg of ascorbic acid to make 100 ml with 2% phosphoric acid (HPO3). Dilute 4 ml of this solution to 100 ml with 2% HPO3 (1 ml = 40 μg of ascorbic acid)
Sample preparation
For strawberries
- Purchase fresh, frozen and canned strawberries products at a local grocery store.
- Sort the berries to remove the ones that are either too small or excessively ripe.
- Place the fresh berries on plastic boxes with perforations.
- There will be four replicates for each treatment administered.
- Tinned strawberries will be refrigerated at a temperature of 273K and about 95% relative humidity for 1 week.
- Frozen strawberries will be refrigerated at a temperature of 253K and about 95% relative humidity for 1 week.
- Fresh berries will be stored at room temperature (21oC)
- Analysis of the fruits will be conducted on 2 day intervals.
For pineapples
- Purchase fresh and canned pineapple products at a local grocery store.
- Remove the cores from the fresh pineapples
- Cut the pineapples into slices of 5 mm thickness and place them in a circular tray of diameter 125 mm and 15 mm height.
- Store half of the fresh pineapple slices at room temperature (294K)
- Place the other half of fresh pineapples in a refrigerator at 273K and around 90% relative humidity
- Conduct tests on 2 day intervals, as the samples remain in storage.
Preparation of sample
For the solid (semi-solid fruit pieces): blend 50 to 100 g of sample with an equal weight of 6% HPO3 and make up an aliquot of the macerate to 100 ml.
Alternatively,
Fruit juices sample: use 10 or 20 ml of sample and make up to 100 ml with 2% HPO3. Filter the mixture
Standard curve
- Dry the test tubes.
- Pipette the requisite volume of standard ascorbic acid solution -1, 2, 2.5, 3, 4 and 5 ml and make up to 5 ml with the requisite amount of 2% HPO3.
- Add 10 ml of dye with a rapid delivery pipette
- Shake and take the reading within 15 to 20 seconds.
- Set the instrument to 100 % transmission using a blank consisting of 5 ml of 2% HPO3 solution and 10 ml of water.
- Measure the red color at 518 nm or a wavelength nearest to the required wavelength using a suitable filter.
- Plot graphs of absorbance against concentration.
Sample
- Place 5 ml of the extract (or less made to 5 ml with HPO3) in a dry test tube.
- Add 10 ml of dye and measure as in standard.
Elimination of interference due to Sulphur Dioxide
The presence of Sulphur dioxide in sample reduces the indophenol dye and thus interferes in ascorbic acid analysis. To eliminate interference in the sample due to SO2, use the formaldehyde condensation procedure given below:
- Put 10 ml of filtrate in a test tube
- Add 1 ml of 40% formaldehyde and 0.1 of HCL
- Keep for 10 minutes and titrate as before
Data analysis
Calculation
Note the concentration of ascorbic acid from the standard curve and calculate the ascorbic acid content in the sample as given below
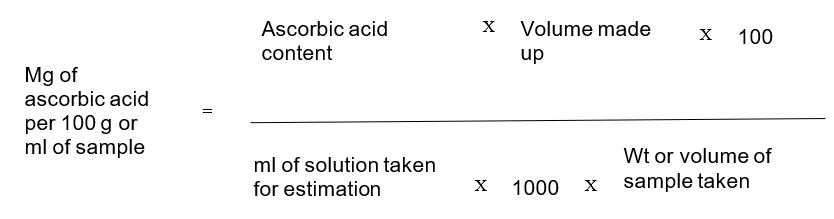
Identify the vitamin C content and draw a graph of absorbance (750 nm) against vitamin C concentration (μg).
- Compare vitamin C content of fresh pineapples and strawberries with the literature values.
- Compare vitamin C content in fresh, frozen and canned pineapples and strawberries
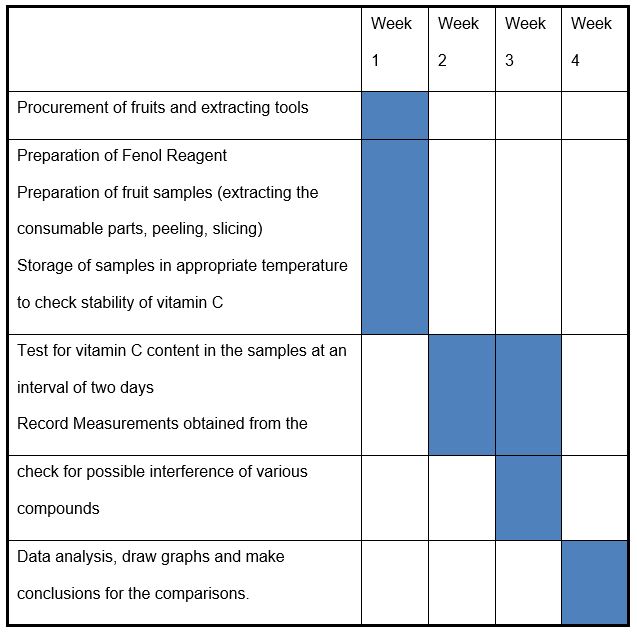
Time plan
Gantt Chart
References
Brevard, P, Marques, K, Renfroe, M, Lee, R & Gloeckner, J 2010, “Differences in antioxidant levels of fresh, frozen and freeze-dried strawberries and strawberry jam”, International Journal of Food Sciences and Nutrition, vol. 61, no. 8, pp. 759–769.
Connell, C, Zoellner, J, Yadrick, M, Chekuri, S, Crook, L & Bogle, M 2011, “Energy Density, Nutrient Adequacy, and Cost per Serving Can Provide Insight into Food Choices in the Lower Mississippi Delta”, Journal of Nutrition Education and Behavior.
Koyuncy, M & Dilmacunal, T 2010, “Determination of Vitamin C and Organic Acid Changes in Strawberry by HPLC During Cold Storage”, Not. Bot. Hort. Agrobot. Cluj, vol. 38, no. 3, pp. 95-98.
Miller, S & Knudson, B 2012, Nutrition & Costs Comparisons of Select Canned, Frozen and Fresh Fruits and Vegetables, Michigan, Michigan State University.
Rababah T, Ereifej K & Howard L 2005, “Effect of ascorbic acid and dehydration on concentrations of total phenolics, antioxidant capacity, anthocyanins, and color in fruits”, J Agric Food Chem vol. 53, pp. 4444–4447
Rickman, J, Barrett, D & Bruhn, C 2007, “Review: Nutritional Comparison of Fresh, Frozen and Canned Fruits and Vegetables. Part I. Vitamins C and B and Phenolic Compounds”, Journal of the Science of Food and Agriculture, vol. 87, no. 7, pp. 930-944.
Vasco C & Ruales J 2008, “Total phenolic compounds and antioxidant capacities of major fruits from Ecuador”, Food Chem, vol. 111, pp. 816–823.