There is no doubt that the world around us is composed entirely of molecules and atoms and that their interaction leads to the formation of new compounds. Recognition of this phenomenon forms the basis for chemical experiments, including those involving the human body. The study of the effects of chemical compounds on the body and in-depth studies of natural internal molecular transformations is a subject of study in biochemistry. For example, one of the known negative effects on a patient’s blood is the forced change in the blood level, which can be initiated by the consumption of strongly acidic or strongly alkaline products. This essay explores this clinical condition in conjunction with a discussion of acid-base interactions.
An uncountable number of existing molecules can be classified into groups to facilitate their identification. For example, according to official definitions, acids are those substances that form hydrogen cations upon dissociation (“Acids and bases,” n.d.). In other words, any acid that exists, whether inorganic or organic, must contain hydrogen. On the other hand, bases are commonly referred to as molecules that usually contain a negatively charged hydroxyl group OH. Examples of some acids (1-3) and bases (4-6) are given below.
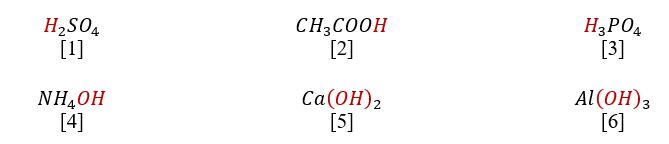
Thus, it is clearly seen that both acids and bases are complex substances, which means that their interaction most likely proceeds on the principle of the double-substitution reaction. By exchanging cations, acid and bases turn into salt and water, as shown below. Consequently, the salt’s nature depends directly on the metal in the base and the acidic residue characteristic of the acid. In addition, this interaction most often leads to the formation of a water molecule, which is why this reaction is commonly referred to as neutralization.
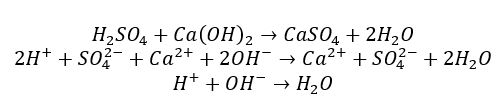
In general, the study of acids and bases is not limited to the neutralization reaction, so it is essential to say what role they play in the surrounding life. For example, the human ventricle naturally secretes hydrochloric acid, lactic acid, and pyruvic acid to maintain functionality. DNA and RNA are nucleic acids, and sulfuric acid is used in industry, including for designing car batteries (“Acids and bases,” 2018). On the other hand, bases can be used as strong bleaches, soaps, the base of potassium-lithium batteries, or to prepare calcium-containing building solutions. Both types of molecules are also permanent tools in the chemical industry for acids or in fine synthesis. The human body is also capable of secreting acids and alkalis to maintain the pH level of the blood.
Each person’s blood is individual, so there is no benchmark for its acidity. However, it can be defined by a range of 7.35-7.45, deviations from which lead to pathologies (Iftikhar, 2019). The mechanism of change in acidity is due to the chemical interaction of opposite molecules, leading to salt and water release. In particular, an excess of alkaline in the blood environment leads to the initiation of neutralization reactions, as a result of which the amount of acid in the blood naturally decreases. If the initial pH in the blood was physiologically normal, then this effect leads to a disturbance of homeostasis. Thus, the consumption of many alkaline foods — mushrooms, avocados, eggs, grains, or vegetables — leads to an increase in pH, called alkalosis. In contrast, when acidic foods such as dairy yogurt, fish, berries, or seafood are consumed, the blood’s pH decreases, and a state of acidosis occurs. Both of these conditions are pathological and have a negative impact on the patient’s health.
References
Acids and bases. (2018). CK-12. Web.
Acids and bases. (n.d.). Lumen Course. Web.
Iftikhar, N. (2019). What’s a normal blood pH and what makes it change? Healthline. Web.