Development and components of the healthy dental pulp
The dental pulp refers to a tissue on an individual’s teeth that is formed by propagating and condensing the neural crest cells of the dental papilla. Trowbridge and Kim (1998) pointed out that a fully grown dental pulp assumes the appearance of embryonic connective tissue. However, the dental pulp’s periphery consists of highly specialised cells.
In addition, the pulp also has microcirculatory components and sensory innervations. Blood flows normally within the dental pulp and provides nutrients and oxygen to the teeth, while still transporting any wastes products for excretion. Bishop and Malhotra (1990) observed that the lymphatic vessels of the dental pulp drain out any excess interstitial fluid.
The cell structure of the dental pulp comprises of fibroblasts and the undifferentiated cells. In addition, the dental pulp also consists of white blood cells, macrophages, and dentritic cells. However, the healthy part of the dental pulp does not have mast cells, with their availability indicating some form of inflammation (Ivar & Karin, 2007).
Functions of the dental pulp
The dental pulp has significant functions. For example, it defends the dentinal tubules from bacterial attack (Nagaoka et al. 1995). There is a remarkable difference in the mode that odontontic and dentinal fluid occupy the dentinal tubules of teeth with vital pulps and those with necrotic pulps. For example, teeth with vital pulps are fully occupied as opposed to the teeth that have necrotic pulps.
According to Linden et al. (1995) and Vongsavan (1993), dentinal fluid refers to ‘positively charged hydrogel’ with the capacity to prevent bacteria from attacking the pulp. In addition, the flow of the dentinal fluid, according to Linden et al. (1995) and Vongsavan (1993), determines the rate at which harmful substances enter into the dentinal tubules from the oral cavity.
Thus, the dentinal fluid dilutes toxins, excretes any invading bacteria, as well as offers defensive support to the dentinal tubules because it has antimicrobial and antibodies to prevent the infection of the dentinal tubules (Trowbridge & Kim, 1998).
The dental pulp has specialised cells, the undifferentiated mesenchymal and odontoblasts cells, that have the capacity to protect the dental pulp from threats of trauma or caries. Such a function can be attributed to the fact that these cells form a hard tissue barrier over the pulp.
In addition, the dental pulp contains the necessary constituents for recognizing and breaking down antigens (Jontell & Bergenholtz, 1992; Jontell et al., 1988; Jontell et al., 1987; Jontell et al., 1998). Alongside the presence of neural, the dental pulp also has proprioceptive capability.
Usually, an individual in need of dental attention might start experiencing excessive dental painful due to sensory stimuli. The presence of proprioceptive receptors within the pulp ensures the detection and reduction of impacts of masticatory forces within an individual’s teeth. The receptors initiate immediate withdrawal reflexes (Byers & Narhi, 1999; Dong et al., 1985).
Evidently, these features add to the defensive role of the dental pulp, as well as the continual protection it provides on an individual’s dentition (Matsutani et al., 2000; Paphangkorakit & Osborn, 1998).
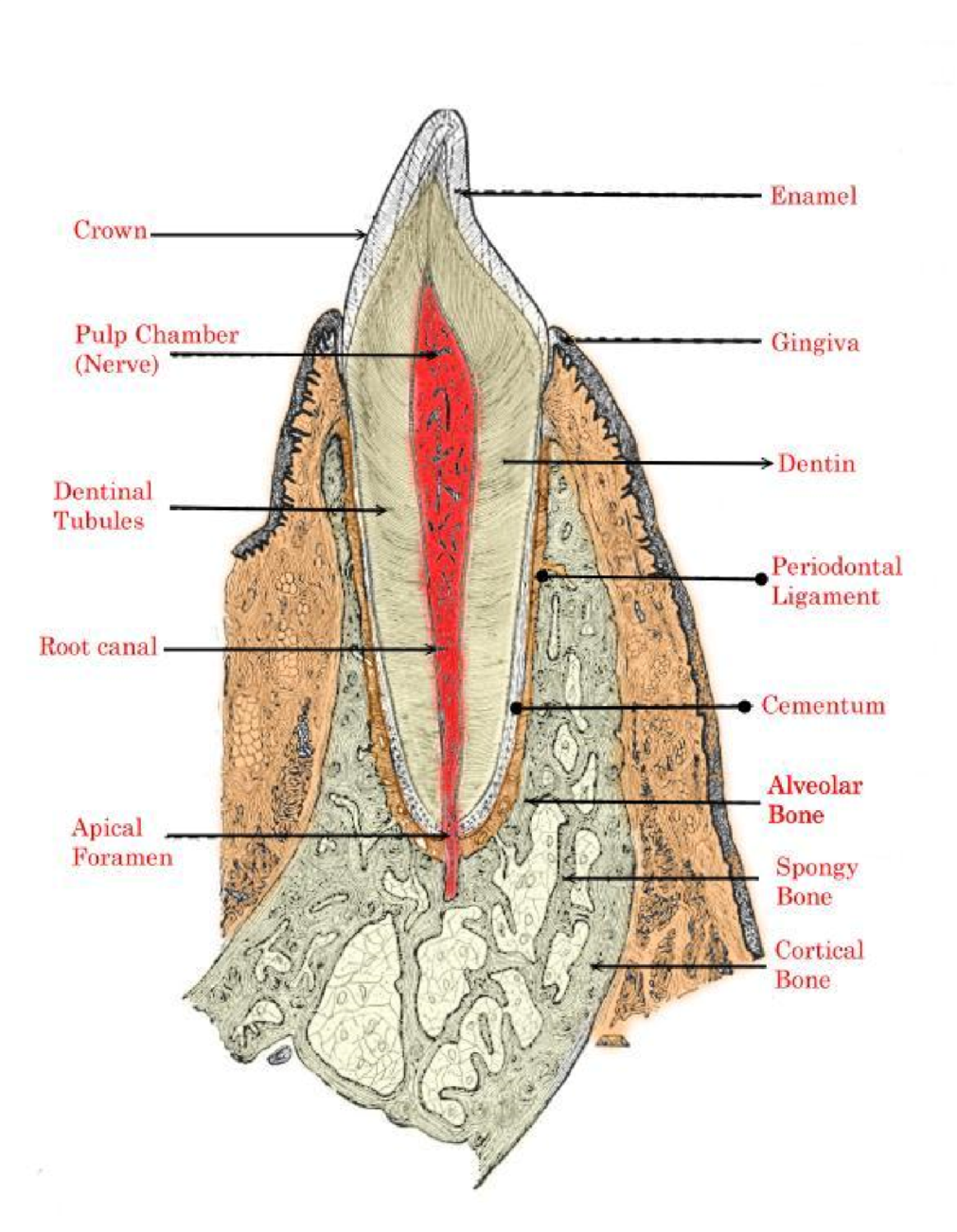
Figure 1: Tooth anatomy.
Innervations of the dental pulp
It is important to understand the features of dental pulp innervations. The trigeminal nerve has the alveolar branches, which supplies neural to the teeth. On the other hand, the lower and upper jaws are supplied by the mandibular and maxillary branches respectively (Abd‐Elmeguid & Yu, 2009). The dental pulp contains a network of tissues that comprise of the sensory trigeminal afferent axons (Byers 1984; Byers & Narhi 1999) and sympathetic fibres (Kim et al., 1989).
The flow of blood within the dental pulp is regulated by the sympathetic fibres, whose source is the cervical sympathetic ganglion. However, the trigeminal ganglion holds the cell bodies of the sensory neurons of the dental pulp (Ingle, 2008).
The apical foramen forms the entry point of numerous axons, after which they branch with respect to the configuration of the blood vessels. When they reach the coronal dentin, the nerve bundles further, branches and moves apart in the direction of the pulp-dentition junction to form the plexus of Raschkow (Dahl & Mjor, 1973; Gunji, 1982).
The unmyelinated C fibres and the myelinated A fibres form the sensory nerve fibre network (Dahl & Mjor, 1973; Gunji, 1982; Miyoshi et al., 1966). However, most of the dentition consists of the myelinated A fibres which are further classified as either Aδ (representing 90% of the A fibres) or Aβ fibres.
The Aδ fibres have little sensitivity as compared to the Aβ in terms of stimulation. On the other hand, the C fibres are found in the pulp proper, as well as have an extension to the cell‐free zone (Byers & Dong, 1983). The excitation threshold of Aδ and Aβ fibres is lower that the excitation threshold of C fibres. For this reason, Aδ and Aβ fibres can adequately respond to a stimulus that is lower than the stimulation threshold of the C fibres (Olgart, 1974).
Vascular supply of the dental pulp
The maxillary artery supplies the dental pulp. The maxillary artery is composed of small arteries and enters the dental pulp via the apical foramen, as well as through accessory foramina. When the small vessels branch from the central arteriole, they form a network of capillaries (Takahashi, 1985).
The formed network of capillaries does not enter the dentinal tubules but moves towards the dental pulp (Takahashi, 1985). The sub-ondontoblastic zone comprises of about 90% of pulpal capillaries, and it is used to provide nutrients to the highly specialised dentin-forming cells.
The venules that are connected to the central pulpal venules help in drainage. There are other shunt vessels that are found within the dental pulp. However, their role in the dental pulp is unknown (Kim et al., 1983; Takahashi et al., 1982), although there are suggestions that the shunts are used in maintaining the flow of blood in the pulp (Kim et al., 1984).
The diseased dental pulp
The duration and type of irritation of the dental pulp determines how the pulp reacts to harmful stimuli. For example, an acute response can be induced in the case of a short-term irritation. During such cases, the inflammatory process can be reversed by removing the irritant.
On the other hand, chronic pulpal inflammation is experienced following long‐term dental pulp irritation, which might result from harsh chemical substances, cracks, tooth surface loss, leaking restorations, and dental caries. The roots of teeth mature in stages, which might determine the pulpal response to stimulation (Cvek et al., 1990; Kling et al., 1986).
Age related changes in the dental pulp
The dental pulp of any individual undergoes different changes as the person ages, (Morse, 1991). Such changes can include root canal calcification, arteriosclerosis, diminished blood supply, and dystrophic mineralisation of the nerve fibres (Bernick, 1967).
Pulp testing of teeth
Indications
According to a review of literature, understanding the state of health of one’s dental pulp is very important (Noblett et al., 1996). For instance, in the light of Ehrmann (1977), vitality tests form the basis of mouth examination. As such, pulp testing of teeth can be carried in case of the following:
Prior to undertaking definitive operative procedures on teeth
A lot of effort has been put on the fact that there can be degenerative pulpal changes on a tooth despite the lack of clinical symptoms or even other radiographic symptoms (Kramer, 1954). In addition, radiographic examination may fail to indicate the loss of cancerous bone (Bender, 1982). For this reason, it is important to carry out pulp tests to find out the actual state of health of the concerned tooth before carrying out any form of treatment (Ehrmann, 1977). Such considerations help to prevent complications in the future.
Diagnosis of pain
Most examinations on dental treatment have showed that orofacial pain is common among dental patients (Ehrmann, 1977). In spite of this, research and analysis in the UK, indicated that the prevalence of orofacial pain, without consideration of headache, of non‐dental origin accounts for 7%, with some of these cases, including chronic pain from other parts of the body (Zakrzewska, 2007). For this reason, it becomes challenging especially in the localisation and diagnosis of pain.
Therefore, effective diagnosis of pain in the trigeminal area can be done through pulp tests (Mumford, 1976; Mumford & Bjorn, 1962). In addition, pulp testing can be used to differentiate between pain of pulpal origin from that of non‐dental origin, as evident in myofacial pain dysfunction syndrome (Duquette & Goebelm, 1973) and referred pain (Harris, 1971).
For the source of pain to be confirmed, there is a need for pulp degeneration signs. In a case whereby the concerned tooth has undergone root canal treatment, it is advisable to look out for an extra sign despite (Ehrmann, 2002).
Investigation of radiolucent areas
In the diagnosis, as well as in the treatment of disorders in teeth, pulp testers are very important (Hare, 1969; Harris, 1971; Klein, 1978). For example, through effective application of pulp testers, apical extension of pulpal pathos may be excluded. After successful exclusion, attention can be focussed on discrimination of any other pathological processes (Harris, 1971; Harris, 1973).
Assessment of teeth following dental trauma
Monitoring of tooth vitality is important in traumatic dental incidents. Such significance is attributable to the fact that some teeth may take several months to respond to pulp testing (Olgart et al., 1988).
In addition, assessing the state of health of the dental pulp, or pulp vitality, helps in the identification of any form of treatment that needs to be carried out on the affected teeth (Roed‐Petersen & Andreasen, 1970), as well as those undergoing surgical trauma. Trauma in this case, can include transplantation procedures (Urbanska & Mumford, 1980), or even sub apical osteotomy (Johnson and Hinds 1969).
Assessment of teeth that have undergone pulp preservation procedures or have required deep restorations
In dental treatment, it is important to confirm the state of the pulpal health from time to time especially for teeth that have undergone extensive restorations or pulp preservation procedures (Cvek, 1978). Pulp tests can be used to primarily indicate the treatment outcome (Dummer et al., 1980; Seltzer et al., 1963).
Evidently, determining the state of the pulp ought to be done early enough to avoid failure of the treatment. If detected later, complications might occur that might be expensive to treat with some resulting to under‐diagnosis and under‐treatment (Tronstad, 1988). Such conditions are highly likely to lead to periradicular periodontitis in abutment teeth (Bergenholtz & Nyman, 1984; Petersson et al., 1999).
Methods
For an ideal pulp testing method, it is important to ensure that the method is simple, objective, standardised, reproducible, non‐painful, non‐injurious, and accurate. In addition, the method ought to be relatively cheap to allow effective assessment of the pulp tissues (Chambers 1982). Nowadays, there are two wide categories of pulp testing methods that can be used in the dental practice. These categories are the pulp vitality and pulp sensibility testers.
Pulp sensibility tests
Pulp sensitivity testers are subdivided into thermal and electric pulp testers. Sensitivity, in this case refers to the ability to feel or perceive sensation. Electric and thermal stimuli are used in pulp sensibility testers. Such stimulus is useful in direct assessment of how the Aδ sensory nerve fibres are reliable within the pulp dentine complex, as well as in indirect determination of the state of pulpal health.
In a case of positive response, such a stimulus shows that there is a possible nerve supply within the dental pulp, while on a secondarily level; it shows that there are vital pulp tissues. This assumption is considered true because the Aδ nerve fibres have a very high sensitivity to hypoxia. Therefore, these fibres can and thus lose reliability in cases of pulp tissue degeneration (Edwall & Kindlova, 1971).
Types of tests and mechanisms of action
Thermal pulp testing
Cold and hot agents can been used in thermal pulp testing, with a variety of cold agents being common nowadays. The agents of thermal pulp testing are used in inducing contraction, which is accompanied by dentinal fluid flow outwardly (Brannstrom, 1986). With respect to the hydrodynamic theory, dentinal fluid generates forces that tend affect the micro-receptors of the Aδ nerve fibres eliciting a form of sensation that ends when the stimulus is removed (Trowbridge et al., 1980).
Nevertheless, cold testing agents have different boiling points, an implication that a considerable reduction of temperature is witnessed whenever the agents are used. Ethyl chloride is an example of cold testing agent that is frequently used in pulp tests, and has a boiling point of ‐4°C.
Other agents such as dichlorodifluoromethane, carbon dioxide, and non-chlorofluorocarbon are also used in thermal pulp testing (Pitt Ford & Patel, 2004). However, there are other agents, such as ice-sticks, that are used in cold‐testing [Figure 3], and bathing the tooth with cold water after isolating it with a rubber dam (Pitt Ford and Patel 2004) [Figure 4].
Gutta-percha points [Figure 5]; bathing the affected tooth with hot water [Figure 6], can be used in pulp testing. However, the gutta-percha method is no longer used because it is difficult to control temperatures and can lead to overheating of the teeth (Narhi 1985), or pulpal damage (Mumford 1964).

Figure 2: Cotton pledget sprayed with a refrigerant agent.
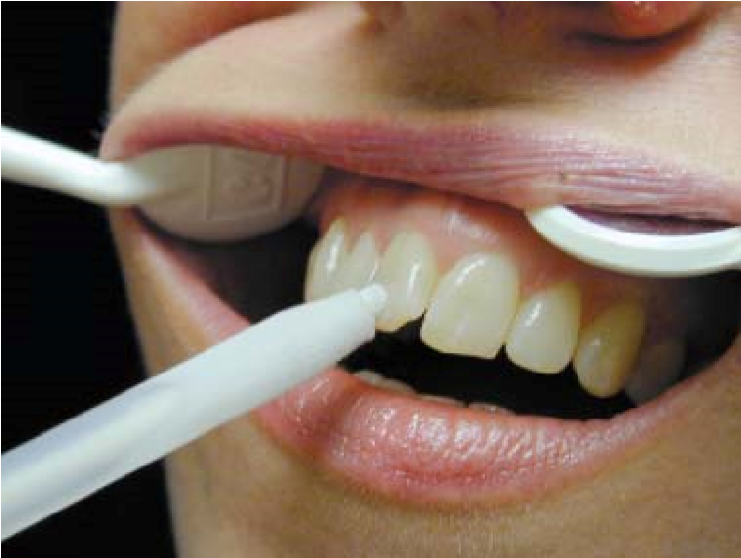
Figure 3: Pulp testing of a tooth using ice‐sticks
Figure 4: Pulp testing of a tooth using a cold water bath following rubber dam isolation
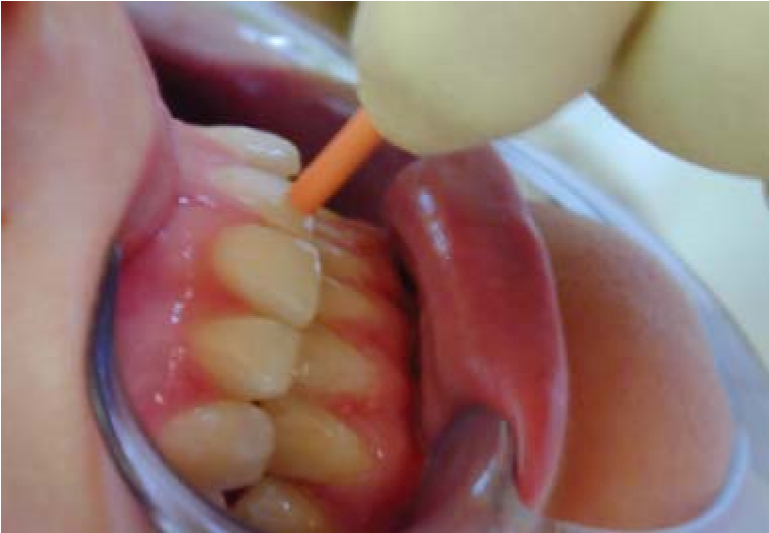
Figure 5: Pulp testing of a tooth using heated gutta-percha
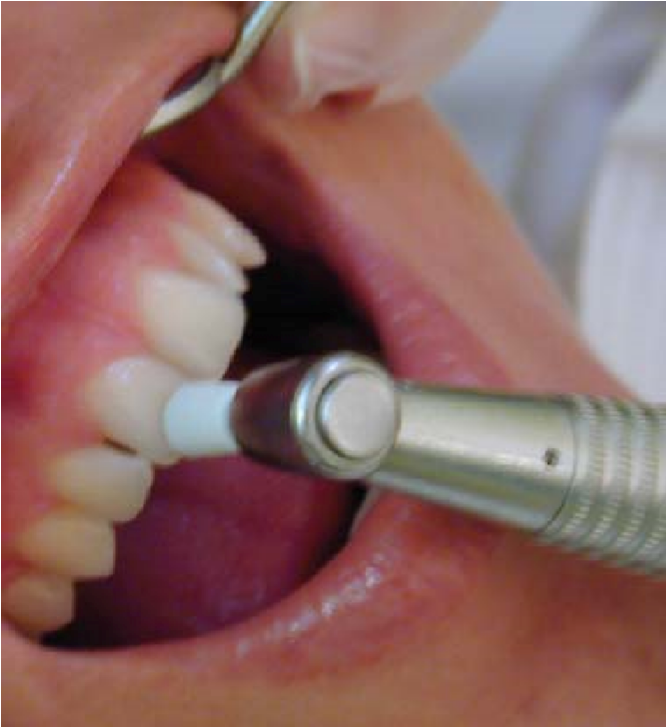
Figure 6: Pulp testing of a tooth using frictional heat generated by a rotating rubber cup.
Electric pulp testing
An instrument that runs on battery is used to perform electric pulp testing. The instrument is used to provide electrical current to the crown of the affected tooth [Figure 7]. A labial hook is used to complete the electric circuit [Figure 8].
However, for the process to be effective, and for maximum current transmission there needs to be a conductive medium between the point of treatment and the tip of the electric pulp tester (Cooley & Robison, 1980; Michelson et al., 1975). Once an electric current is initiated, an action potential is generated that is followed by the depolarization occurring within the dentinal fluid (Pantera et al., 1993).
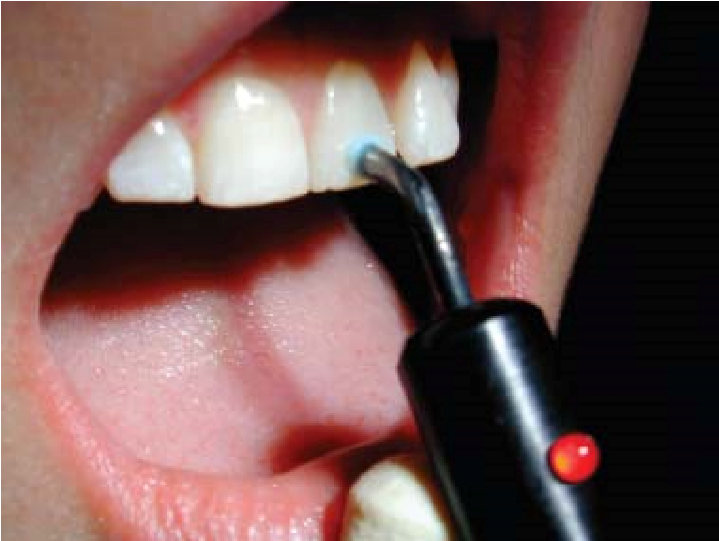
Figure 7: Pulp testing of a tooth using an electric pulp tester
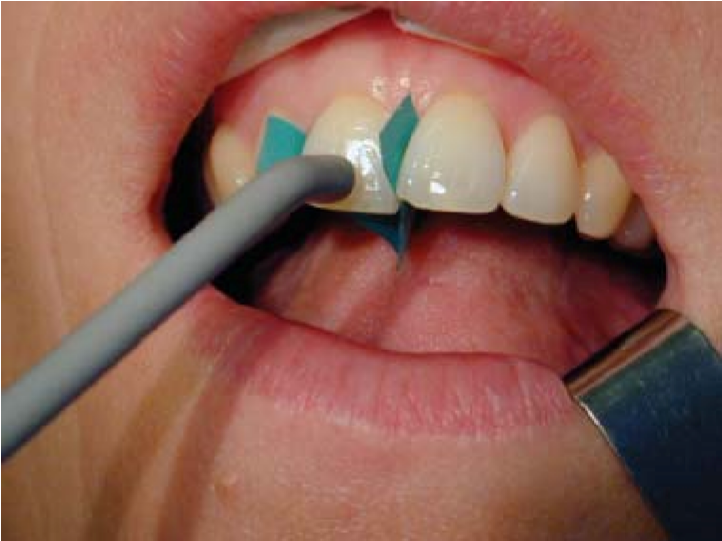
Figure 8: Electric pulp testing of an upper right central incisor isolated from adjacent teeth by rubber dam strips.
General limitations of pulp sensibility testing methods
Even though pulp sensibility testers may not comply with all the features of an ideal testing method, they can be quite effective if properly applied. However, it is important to consider the following limitations:
Assessing nerve viability rather than pulp vitality
One of the first steps of assessing the state of the dental pulp health is to examine the flow of blood within the dental pulp (Bhaskar & Rappaport, 1973; Chambers 1982; Fratkin et al., 1999). As such, the ‘pulp vitality tester’ has the implication of an analytic method with the capability to directly assess the vascularity of the dental pulp.
On the other hand, pulp sensibility tests can be used in determining whether or not a nerve supply is reactive (Bhaskar & Rappaport, 1973; Ehrmann, 1977; Johnson & Hinds, 1969). In most of the cases, pulp sensitivity tests are considered as proxy markers (Ikeda & Suda, 1998; Yanpiset et al., 2001). However, thermal and electrical tests often give false responses, thus making tests unreliable in the case of damage on the pulp vasculature (Radhakrishnan et al., 2002).
Lack of correlation with the histological condition of the dental pulp
Findings from studies on several pulp testing methods do not show any correlation when compared with the history of the dental pulp (Barker and Ehrmann 1969; Lundy and Stanley 1969; Marshall 1979; Matthews et al. 1974; Mumford 1967). Nevertheless, a significant relationship has been identified between necrotic pulp tissues and the negative responses observed during pulp testing (Lundy & Stanley, 1969; Marshall 1979; Seltzer et al., 1963).
Lack of objectivity
There has been a lot of debate on the role of an individual’s response to pulp sensitivity, with some scholars pointing out that such response is very objective (Ingle & Beveridge, 1976), and others disagreeing with reasons based on the nature of pain (Degering, 1962; Ehrmann, 1977; Stark et al., 1977).
However, such misunderstanding can be overcome by setting up a control tooth during the testing process (Chilton & Fertig, 1972). In spite of this, the control tooth may not be useful in the event of a wrong and inaccurate method (Seltzer et al., 1963). In addition, the degree of neural response does not necessary imply that there is a pathological condition (Mumford, 1976).
Lack of reproducibility
Research and analysis in dental practice has showed that different individuals respond differently to pulp tests, depending on the days, and time of the day, which is attributable to the diverse emotional conditions among patients (Grossman, 1978). In addition, available types of electric pulp testers have different degree of accuracy (Cooley & Robison, 1980; Matthews et al., 1974).
Unpleasant sensation
The stimulation of the pulpal nerves pulpal sensibility testing, elicits individuals to respond differently. Several researchers have showed that stimulation of the pulpal nerves leads to pain (Naylor, 1964; Greenwood, 1973).
On the other hand, other researchers have countered such proposition arguing that there are several instances patients get different sensations other than pain (Mumford & Newton, 1969). In spite of such arguments, the sensation that an individual experience as a result of pulpal stimulation is often unpleasant. In addition, this unpleasant sensation often establishes a premature response on the affected patient (Cooley & Robison, 1980).
Effect of distribution of teeth in the oral cavity
Multi-rooted teeth have often been hard to assess the state of their pulpal health as compared to single‐rooted teeth. There is a high possibility of positive response for a tooth that has vital pulps as opposed to neurotic pulps (Peters et al., 1994).
Effect of maturation status of the tooth
During the early development of teeth up to about 5 years, there is incomplete neural supply within the dental pulp (Bernick 1969). Theoretically, during such development stages, it is of no use to carry out pulp sensitivity tests. This can be attributed to the fact that pulp sensitivity tests on immature teeth often register no response (Klein 1978) to positive response, but shows a negative high value when using the EPT (Dummer et al., 1980; Grossman, 1978).
However, according to a study carried out by Fulling and Andreasen (1976), the EPT recorded no response for immature canines, while a response was observed for other teeth at a higher threshold value. As such, there was an inverse relation between the threshold value and the degree of tooth maturation. In addition, it was evident that for immature teeth, carbon dioxide was a better substitute for EPT (Fulling & Andreasen, 1976).
Effect of tooth condition
When assessing the state of health of the dental pulp for a tooth that was restored by covering the crowns with a metal, carbon dioxide is better testing agent (Trope & Sigurdsson, 1998). In addition, the clinician can consider the use of dental instruments like sickle probes, in cases where there is fear of contact with gingival tissues, as well as when there is a need to avoid subsequent false‐positive responses (Pantera et al., 1992).
Effect of trauma
Dental pulp blood vessels are more resistant to damage than the pulpal nerves (Bhaskar & Rappaport, 1973). During dental treatment, the neural undergoes temporary disruption, which can recover within a period of six months (Zadik et al., 1979). For this reason, it is unreliable to use pulp sensitivity testers in the assessment of the state of pulpal health of teeth, because the process is accompanied by traumatic dental injuries (Bhaskar & Rappaport, 1973; Ehrmann, 1977; Teitler et al., 1972; Zadik et al., 1979).
Effect of orthodontic treatment
There are numerous changes that occur during pulp sensibility testing of orthodontically treated teeth (Hamersky et al., 1980). For this reason, the results from such a procedure cannot be relied upon (Burnside et al., 1974; Hall & Freer, 1998). Orthodontically treated teeth have showed characteristics of responding to electrical pulp testers at a high threshold of about nine months (Cave et al., 2002). As such, better agents such as carbon dioxide can be used in such cases to assess the state of pulpal health (Cave et al., 2002).
Pulp vitality tests
Early stages within the dental pulp have been cited to play a major role in pulpal inflammations (Baumgardner et al., 1996; Kim, 1990). For example, the flow of the blood within the dental pulp provides oxygen and nutrients to the dental pulp. As such, if the flow of blood is altered, the rate at which the pulp gets oxygen and other important nutrients is interfered with.
As such, the flow of blood within the dental pulp is a very important aspect of the pulp. Thus, whereas the use of pulp sensibility testers is very important in the provision of significant information about dental diagnosis, it is important to assess the state of vascular supply for proper determination of the pulpal health (Fratkin et al., 1999; Radhakrishnan et al., 2002). 2.2.2.2.1 Types of tests and mechanisms of action
There are different methods available nowadays to ascertain the nature of the flow of pulpal blood. Based on their significance, these methods have become common within the dental practice. Some of the commonly used methods are highlighted below:
Laser Doppler flowmetry (LDF)
The Laser Doppler flowmetry (LDF) method became common within the dental practice in the late eighties, and preferred for its non-invasive characteristics when used in the measurement of the flow of pulpal blood within an individual’s teeth (Gazelius et al., 1988; Gazelius et al., 1986) [Figure 9].
This method use the technology of infra-red beam propagated from a laser source, and directed to the area tooth under investigation. As such the light is allowed to move via the dentinal tubules towards the dental pulp (Matthews & Vongsavan, 1993) [Figure 10].
After reaching the dental pulp, red blood cells and other tissues within the pulp absorb the light. In the case of a back-scattered light, Doppler shifting can never occur unless there is an interaction between the circulating red blood cells and photons of light. If the interaction occurs, a portion of the light is send back to the photo detector for analysis (Ingolfsson et al., 1994). The output signal is viewed in the form of flux, and a concentration of blood cells (Gazelius et al. 1988; Olgart et al. 1988), as shown in Figure 11.

Figure 9: Laser Doppler Flowmeter (LDF) [Moor Instruments, Axminster UK].
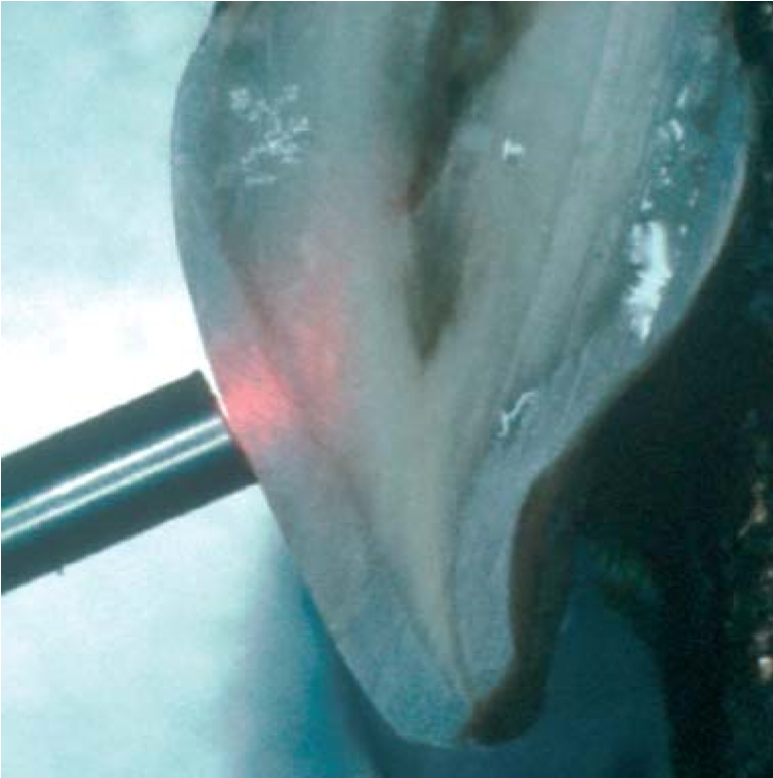
Figure 10: Application of LDF probe to the facial surface of a sectioned tooth reveals the passage of light through enamel prisms and dentinal tubules to reach the dental pulp.
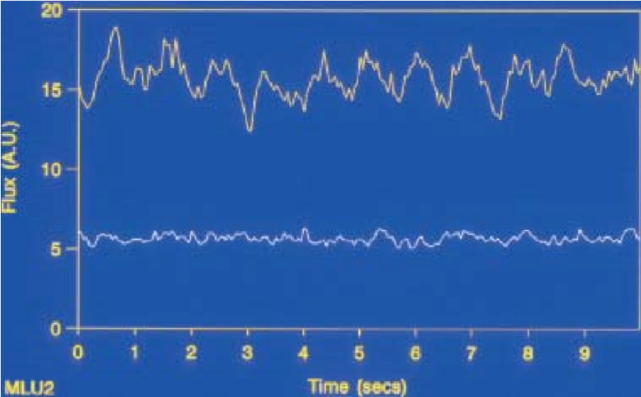
Figure 11: Signals recorded by LDF. A diagram showing vital tooth is shown by the line at the top; a non‐vital tooth appears at the bottom.
Laser Doppler Flowmetry has been considered semi-quantitative, objective, reliable, reproducible, and non-invasive when used in the measurement of pulpal blood flow (Gazelius et al., 1988; Gazelius et al., 1986; Olgart et al., 1988; Raab et al., 1988; Ramsay et al., 1991a; Ramsay et al., 1991b; Sasano et al., 1989; Wilder‐Smith, 1988). In spite of such features, there are other factors that may affect the suitability of LDF in measurement of pulpal blood flow. Such variables are outlined below:
Laser beam characteristics
The bandwidth that is used in any form of dental pulp examination is very important since it influences the flux values. Therefore, clinicians ought to choose the right bandwidth, with a narrow bandwidth of 3.1 KHz being preferred (Roebuck et al., 2000; Odor et al., 1996a; Odor et al., 1996b). On the other hand, the laser source ought to be relatively longer, preferably 810 nm because such a length allows in-depth penetration of the pulpal vasculature (Odor et al., 1996a; Odor et al., 1996b).
Probe position and non‐pulpal signals
In cases where the probe was next to the gingival margin, several studies indicated high flux values (Hartmann et al., 1996; Ramsay et al., 1991a), which is attributed to a continuous flow of blood signals within the non-pulpal tissues that are close to the probe. Some of these tissues include the tongue, lips, the periodontium, and gingival tissues (Hartmann et al., 1996; Ikawa et al., 1999; Polat et al., 2004; Polat et al., 2005; Soo‐ampon et al., 2003; Vongsavan & Matthews, 1993).
The ideal distance from the gingival margin should be 2‐3 mm, as such a distance minimises the contamination of signal. In addition, such a distance minimises signal noise, and is suitable in the provision of an output that is recognizable (Roebuck et al., 2000; Vongsavan & Matthews, 1996).
In other cases, reduction of the signals recorded can be achieved through a considerable isolation of the affected tooth by the use of an opaque rubber dam, which may also apply to cases involving vital teeth (Soo‐ampon et al., 2003; Kijsamanmith et al., 2011).
Probe design
The optical separation distance is used to determine the probe design, and has a significant effect on the recorded signal. In addition, the separation distance is very useful in determining the concentration and flux values of the recorded signal (Roeykens et al., 1999).
Probe holder characteristics
A flux artefact is produced as a result of loud sounds, and extraneous noises (Musselwhite et al., 1997; Mesaros & Trope, 1997). Such artefacts can be reduced by the use of opaque putty (Mesaros & Trope, 1997).
Tooth type
Sasano et al. (2005) showed that LDF was unreliable in getting signals in cases involving from thicker teeth, through the use of a low-power laser beam. From this study, it was evident that the thick layer around the teeth’s dentition prevents photons from penetrating deeply into the pulp tissues (Sasano et al., 2005).
Tooth surface imperfections and discolouration
The surface of the tooth can have several imperfections such as cracks (Hartmann et al., 1996), as well as light‐absorbing pigments (Heithersay & Hirsch, 1993). Such imperfections tend to interfere with the signal output.
Use of medications
Factors such as the use of anaesthetic agents (Chng et al., 1996), neurogenic mediators (Olgart et al., 1989), vasoactive substances (Okabe et al., 1989), and smoking (Johnson et al., 1993) influence the recorder signal significantly.
Pulse Oximetry (PO)
Pulse Oximetry (PO) refers to a non‐invasive and objective method that is used in the measurement of the level of oxygen saturation in the blood. It uses a photo detector to identify the amount of oxygen absorbed, as well as calculates the saturation level of the absorbed oxygen (Noblett et al., 1996; Raab et al., 1988; Schnettler & Wallace, 1991).
Transmitted light photoplethysmography (TLP)
This method is used in the assessment of the flow of blood within the dental pulp of humans, as well as of animals (Daley et al., 1988; Miwa et al., 2002; Lindberg et al., 1991).
Dual wavelength spectrophotometry (DWLS)
Dual wavelength spectrophotometry, commonly abbreviated as DWLS, is an example of non‐invasive technique that makes use of a fibre optic probe in the transmission of visible light of dual wavelengths (760 and 850 nm) (Nissan et al. 1992), in the assessment of whether or not there is any oxygenated blood. There are hopes that DWLS will be useful in the future as a pulp testing method (Nissan et al. 1992).
Thermography and tooth surface measurement techniques
Studies have showed that teeth that have a healthy supply of blood have warmer surfaces as compared to those that have a degenerated blood supply. Such a condition explain why thermo-graphic imaging are used in the measurement of blood flow in the dental pulp (Pogrel et al., 1989; Kells et al., 2000b; Kells et al., 2000a). For instance, electric thermometers that can be attached onto the dental probes (Fanibunda, 1986b; Fanibunda, 1986a), as well as liquid crystals with the ability to give different colours when subjected to heat are examples of tooth‐surface measurement techniques (Howell et al., 1970).
Limitations of pulp vitality testing methods
There are several limitations of using pulp sensitivity testers. However, most of these limitations do not apply in the case of pulp vitality testers. For example, the methods used in pulp vitality testing are often objective. For this reason, the need to assess a patient’s response is eliminated, which has been considered to be a source of errors especially when examining children, as well as disabled patients.
In addition, research and analysis has showed that pulp vitality testers do not inflict any pain on the patient, and that they are non-invasive. In spite of this, there are a few limitations that are associated with pulp vitality testers. For example, they use highly complex thermo-graphic imaging technique (Kells et al., 2000b).
Similarly, these testers require clinicians to adhere to the technique for pulp vitality testers as in PO, failure to which it becomes impossible to obtain reliable results. In addition, conformity is necessary when using pulp vitality testers because the size of the teeth must be in line with the size of the photo detector sensor used, as well as the size of the anatomical contour. In addition, it is important to ensure that the photo detector and the light‐emitting probe are strategically placed (Vaghela & Sinha, 2011).
According to earlier discussion (section 2.2.2.2.1.1), it was observed that LDF can change with respect to light and movement. For this reason, an opaque putty order is necessary to avoid the effect of sunlight and movement on the LDF. For example, when investigating LDF, PO, or TLP next to a gingival margin, there are high chances of errors due to the effect of external signals. Nevertheless, signal contamination is low in TLP as opposed to LDF (Miwa et al., 2002).
Therefore, the ideal distance from the gingival margin should be 2‐3 mm since such a distance minimises the contamination of signal. However, pulp vitality testers are not suitable for use in the assessment of the pulpal health for teeth that underwent heavy heavily restorations and crowning. In addition, such methods are not reliable in assessing the state of the pulpal health of teeth with vital apical pulps.