- Introduction
- Use of Biomimetics in Restorative Dentistry
- Stem Cell Therapy
- Biomimetics and Pulp Transplant
- Injectable Scaffold Delivery and Biomimetics in the Last Decade
- 3D Cell Printing and Biomimetics in Dentistry
- Bioengineered Tooth and Biomimetics
- Gene Therapy: A Novel and Promising Application of Biomimetics in Dentistry
- Conclusion
- References
Introduction
In the vast field of medicine, biomimetics refers to the study of the functions of naturally occurring biological organisms and materials. The aim of such studies is to mimic the properties of these elements. The imitation can be in the form of human-made processes or systems. Biomimetics is derived from two Greek words. The two are bios, which means life, and mimesis, which means imitation (Kottoor 2013). The term was first used by Ottoschmit during the late 1950s. The main challenge in biomimetics is to find biomimetic materials that can be integrated with human tissues at the cellular level (Kottoor 2013). Technological advancements have made this possible to some extent. Scientists are hoping to make more breakthroughs in the near future.
Use of Biomimetics in Restorative Dentistry
The British Society for Restorative Dentistry provides a working definition of this concept. Restorative dentistry is defined as the study, analysis, and treatment of diseases occurring within the oral cavity. The treatment is concerned with the strength and functionality of human teeth. To understand how biomimetics is applied in dentistry, one must have basic knowledge of the tooth, specifically the pulp and the dentin regions (figure 1). According to Mauth et al. (2007), the pulp and the dentin have a common embryonic origin, which is the dental papilla. The two parts share a strong bond throughout the lifetime of the tooth. The relationship is referred to as the pulp-dentin complex (Mauth et al. 2007).
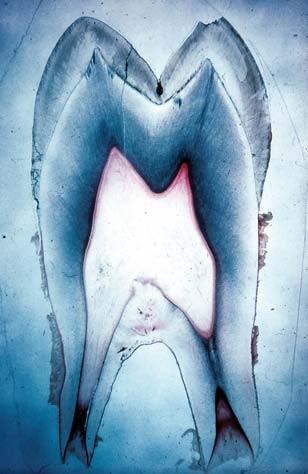
Cm regenerative dentistry. They include regeneration of pulp tissues and stem cells.
Stem Cell Therapy
Stem cells are known for their ability to multiply. They are broadly categorised into two. They include adult and embryonic stem cells. Both possess the self-division property. In addition, they both grow in-vitro. It is the adult stem cells that are commonly used in medical practices. The reason is mainly due to the low number of ethical issues associated with the practice (Kottoor 2013).
Stem cell therapy is considered to be one of the simplest ways of initiating cell regeneration process. Cells with regenerative properties, in this case the postnatal stem cells, are injected into the root canal of the damaged tooth. The most commonly used postnatal stem cells include SHED, DPSCs, and SCAP. The first category is made up of stem cells extracted from the human ‘milk’ teeth. DPSCs, on the other hand, are extracted from the dental pulp. On their part, SCAP are sourced from apical papilla (Garcia-Godoy & Murray 2006). The DPSCs are the most preferred stem cells due to their ability to regenerate a dentin. The resulting dentin is composed of mineralised matrix with tissues. It is also vascularised in a manner that is similar to what is found in a normal human dentin (Kottoor 2013).
The formation of dentin is similar to that of bone. During the formation process, inorganic calcium phosphate is deposited in a matrix formed by a single layer of cells. On the crown area of the tooth, the dentin is covered by the enamel and by the centrum on the root area. According to Mauth et al. (2007), the dentin is not remodelled at any stage during the lifetime of the human. As such, the pulp chamber eventually runs out of pulp as the tooth grows old. The dentin is made up of 50 percent minerals. On the other hand, 30 percent is organic matter. Water takes up the remaining 20 percent (Nakahara et al. 2009). The matrix formed contains penetrating and extremely narrow tubes. The tubal formations are called dentinal tubules (refer to figure 2 below). It is these dentinal tubules that accommodate the odontoblastic process.
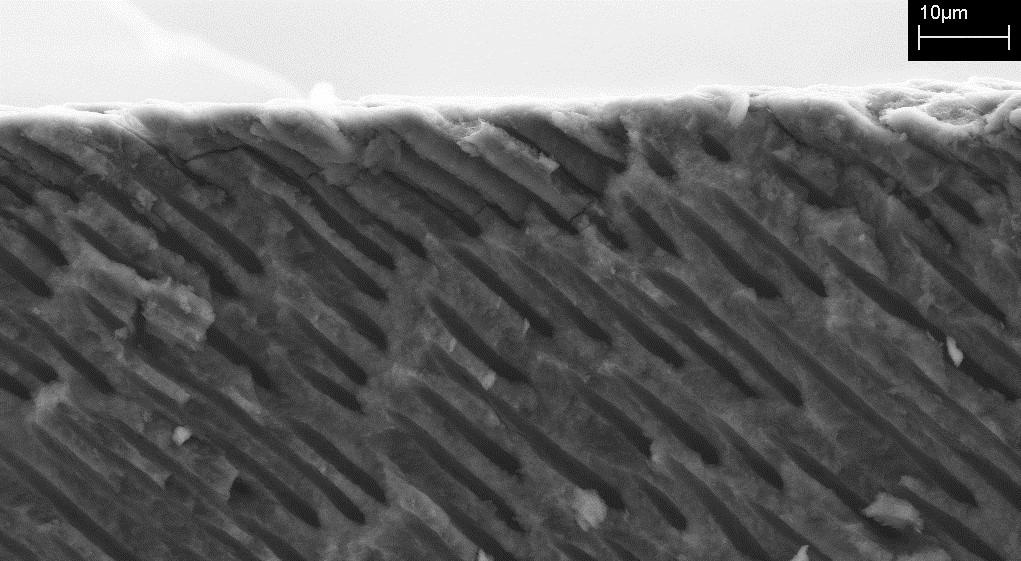
Odontoblasts are the most prominent cells in the dentin-pulp complex (Mauth et al. 2007). They are responsible for the formation of the dentin. Odontoblastic process is their transition into dentinal tubules. In the early stages of tooth formation, these cells form the predentin. Predentin is an organic matrix whose main component is collagen. After the eruption of the tooth above the gum, secondary dentin is slowly deposited in small portions. The process continues for the entire life of the tooth (Yu et al. 2007).
The formation of tertiary dentin is triggered by exposure to stimuli. The odontoblastic cells responsible for the formation of tertiary dentin are from the stem cells in the pulp. It is these postnatal cells that make up the dental pulp stem cells (DPSC), which are used in dentistry biomimetics.
Biomimetics and Pulp Transplant
Pulp transplant is another technological advancement with regards to the status of biomimetics and dentistry within the last decade. The dental pulp is a highly vascularised tissue. It is located at the root canal of the tooth. Its main function is to strengthen the tooth. To this end, it responds to bacterial invasion, injury, and mechanical stimuli. It promotes regeneration of other tissues (Ravindran & George 2015: Ravindran & George 2014). Although the dental pulp promotes regeneration among other tissues, it does not regenerate itself. The reason is mainly due to its anatomical arrangement (Berkovitz, Holland & Moxham 2009; Ravindran et al. 2014).
The common treatment for damaged dental pulp is root canal therapy. Here, the unhealthy dental pulp is removed. The remaining void chamber is then disinfected. The pulp chamber is then filled with gutta percha. Gutta percha is a synthetic compound that does not support regeneration of tissues. As a result, the tooth loses its sensitivity and eventually dies. Root canal treatment deals efficiently with most of the conditions related to teeth. However, it leads to secondary infections due to the inability of the tooth to respond to bacterial attacks. However, this problem can be overcome with the help of regeneration pulp tissue. The method involves removing the dysfunctional pulp tissues and replacing them with working pulp tissues. Within the last decade, most practitioners have preferred using DPSC tissues. The reason is that the tissues are capable of multi-lineage differentiation (Huang et al. 2010; Kerkis et al. 2006; Levorson et al. 2014).
The process of removing damaged pulp tissues and replacing them with newly created and healthy ones is known as regenerative endodontics (Deepak & Reddy 2014). However, most practitioners feel that the focus should be on how to regenerate the damaged dysfunctional pulp tissues. Replacing them is not the best alternative. The reason is that practitioners are having difficulties designing biometric materials used in the fabrication process. In regenerative endodontics, the new pulp tissue is made through tissue engineering triad. The concept was introduced by Langer and Vacanti in 1993. It has been refined significantly within the last decade (Desgrandchamps 2000). Prescott et al. (2008) describe how a team of experts engineered pulp tissue using the triad and dental pulp stem cells. The experts also used Dentim Matrix 1, a growth factor, and Collagen Scaffold. They tested the tissue on mice. The team concluded that that the tissue engineered triad can form an organised matrix (Luo & Shoichet 2004). The matrix resembles the one found in pulp tissue.
Dental pulp implant is associated with a number of challenges. The problems are compounded when implanting tissue into a pulp canal with a blood supply that is coming from the apical end only. Nano scale technology, which is still under development, is required in such cases to create channels through the pulp. According to Kottoor (2013), research has intensified within the last decade with aims to create scaffold systems that would enhance angiogenesis. The process would promote vascularisation. The scaffolds are injected with growth factors that lack platelets (Nakahara et al. 2009; Oshima et al. 2011; Rosa et al. 2013).
Injectable Scaffold Delivery and Biomimetics in the Last Decade
It is another technological advancement in the last decade. In most parts of the body, engineered tissues provide physical support to cells used in bone formation. However, dental pulp is not required to provide any physical support to the tooth. The situation makes it possible for the pulp to be administered in a soft three-dimensional scaffold tissue (Deepak & Reddy 2014). A biomaterial called hydrogel is commonly used for these purposes. Some of the desired properties of this material include its ability to be conveyed to the root canal through injection. It also has non-invasive properties. Theoretically, hydrogels enhance pulp regeneration. They do this by providing substrates for cell proliferation and differentiation into organised tissue structures (Sanjana & Fuller 2004). The use of hydrogels has not yet been perfected. Tests reveal that most of them lack total control over the formation and development of tissues. However, the limitation is adequately managed due to recent advancements in the formulation of hydrogels (Desgrandchamps 2000). Researchers are trying to make these tissues more practical for clinical application. They achieve this by photopolymerising them to form rigid structures once in a tissue site (Luo & Shoichet 2004).
3D Cell Printing and Biomimetics in Dentistry
It is one of the most optimistic researches being undertaken in tissue engineering. It involves the use of three-dimensional scaffolds to support and guide the cells in the pulp during their initial stages of growth (Thesleff & Tummers 2003). The scaffolds are porous and have a designed pore organisation that interferes with the normal migratory pattern of cells (Sanjana & Fuller 2004). It is important to regulate the migratory patterns of the cells and interactions between them. The objective can be achieved through the use of 3D forms produced according to a predesigned structure. A device resembling an ink-jet is used to dispense the layers of cells trapped in hydrogel to form the structure of the tooth pulp tissue (Kottoor 2013).
Bioengineered Tooth and Biomimetics
It is one of the milestones hoping to be achieved in the field of regenerative therapy. It is based on the concept of transplanting regenerative tooth material and providing it with the right growth factors. The intervention supports the growth of a new tooth. A fully functioning tooth replacement has already been reported within the last decade. Ikeda et al. (2009) achieved the transplant by placing a bioengineered tooth germ into the alveolar bone. The experiment was carried out on an adult mouse with a lost tooth. The tooth that developed on the alveoli bone had the correct structures and composition. However, the researchers could not identify a strategy to manage the size of the tooth. The resulting structure was smaller than expected. However, recent experiments have established that the size of the tooth, as well as the width of the crown and number of cusps, can be controlled (Oshima et al. 2011). The control can be achieved through cell manipulation (Oshima et al. 2011).
Gene Therapy: A Novel and Promising Application of Biomimetics in Dentistry
Gene transfer is not entirely new in clinical practice. It has been around for over 10 years. It was first witnessed when two children were diagnosed with immunodeficiency resulting from genetically inherited low levels of the ADA enzyme (Krasnodembskaya et al. 2010). To treat them, adenosine deaminase gene was transferred into the patients’ own lymphocytes. The lymphocyte cells were afterwards returned to the patients’ bodies (Karaoz et al. 2011). Both children survived. However, studies have been unable to confirm whether or not the survival of the children was as a result of the gene transfer. The reason is that they were receiving conventional treatment at the same time (Thesleff & Tummers 2003).
During gene transfer, the viral vectors are changed to reduce chances of infecting the recipient. According to Kottoor (2013), the vectors developed today include retroviral, herpes simplex virus, lentivirus, adenoviral, and adeno associated virus. Non-viral delivery systems, on the other hand, involve the use of plasmids, peptides, and DNA-ligand complex (Magne & Belser 2002). Gene therapy can be achieved through two strategies. The first is in-vivo. The other is ex-vivo. In-vivo is where the genes are delivered systematically into the blood or into the tissue through injections. BMP genes are delivered this way into the pulp (Kottoor 2013). Ex-vivo is slightly different. The first step involves manipulating cellular structures in-vitro. They are then relocated to the regeneration locale.
The challenges associated with gene therapy involve, among others, the costs involved (Berkovitz, Holland & Moxham 2009). For the proposed project to succeed, it should be made a cost effective and affordable therapy. The reason is that not many people can afford conventional genetic treatments given that they are relatively expensive. Gene therapy through biomimetics should also provide long term solutions to dental conditions.
Conclusion
It is a fact that biomimetics is the new face of dentistry around the globe. People and organisations are increasingly dedicating their resources to this field. As a result, progress is clearly evident. Positive results have been generated within the last decade. However, humanity is a long way from the realisation of the dream to fully exploit the benefits of biomimetics. The limitations persist even in the face of the scientific progressions made in the recent past. However, stakeholders still hope that this is the answer they have been looking for.
References
Berkovitz, B, Holland, G & Moxham, B 2009, Oral anatomy, histology, and embryology, 4th edn, Mosby, London.
Deepak, V & Reddy, K 2014, ‘Biomimetics in dentistry’, Indian Journal of Research in Pharmacy and Biotechnology, vol. 2 no. 5, pp. 2320-3471.
Desgrandchamps, F 2000, ‘Biomaterials in functional reconstruction’, Current Opinion in Urology, vol. 10 no. 3, pp. 201-206.
Garcia-Godoy, F & Murray, P 2004, ‘Stem cell responses in tooth regeneration’, Stem Cells and Development, vol. 13 no. 3, pp. 255-262.
Huang, G, Yamaza, T, Shea, L, Djouad, F, Kuhn, N, Tuan, R & Shi, S 2010, ‘Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model’, Tissue Engineering Part A, vol. 16 no. 2, pp. 605-615.
Ikeda, E, Morita, R, Nakao, K, Ishida, K, Nakamura, T, Takano-Yamamoto, T, Ogawa, M, Mizuno, M, Kasugai, S & Tsuji, T 2009, ‘Fully functional bioengineered tooth replacement as an organ replacement therapy’, Proceedings of National Academy of Science of the United States of America, vol. 106 no. 32, pp. 13475-13480.
Karaoz, E, Demircan, P, Saglam, O, Aksoy, A, Kaymaz, F & Duruksu, G 2011, ‘Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells’, Histochemistry and Cell Biology, vol. 136 no. 4, pp. 455-473.
Kerkis, I, Kerkis, A, Dozortsev, D, Stukart-Parsons, G, Gomes-Massironi, S, Pereira, L, Captain, A &Cerruti, H 2006, ‘Isolation and characterisation of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers’, Cells Tissues Organs, vol. 184 no. 3, pp. 105-116.
Kottoor, J 2013, ‘Biomimetic endodontics: barriers and strategies’, Health Sciences, vol. 2 no. 1, pp. 1-11.
Krasnodembskaya, A, Song, Y, Fang, X, Gupta, N, Serikov, V, Lee, J & Matthay, M 2010, ‘Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37’, Stem Cells, vol. 28 no. 12, pp. 2229-2238.
Levorson, E, Hu, O, Mountziaris, P, Kasper, F & Mikos, A 2014, ‘Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, Part 2: construct devitalisation and determination of chondroinductive capacity’, Tissue Engineering Part C Methods, vol. 20 no. 4, pp. 358-357.
Luo, Y & Shoichet, M 2004, ‘Light-activated immobilisation of biomolecules to agarose hydrogels for controlled cellular response’, Biomacromolecules, vol. 5, pp. 2315-2323.
Magne, P & Belser, U 2002, Bonded porcelain restorations in the anterior dention: a biomimetic approach, Quintessence Publishing Company, Chicago.
Mauth, C, Huwig, A, Graf-Hausner, U & Roulet, J 2007, ‘Restorative applications for dental pulp therapy’, Topics in Tissue Engineering, vol. 3, pp. 20-32.
Nakahara, H, Misawa, H, Hayashi, T, Kondo, E, Yuasa, T, Kubota, Y, Seita, M, Kawamoto, H, Hassan, W, Hassan, R, Javed, S, Tanaka, M, Endo, H, Noguchi, H, Matsumoto, S, Tanaka, K, Tashiro, Y, Nakaji, S, Ozaki, T & Koboyashi, N 2009, ‘Bone repair by transplantation of hTERT-immortalised humanmesenchymal stem cells in mice’, Transplantation, vol. 88 no. 3, pp. 346-353.
Oshima, M, Mizuno, M, Imamura, A, Ogawa, M, Yasukawa, M, Yamazaki, H, Morita, R, Ikeda, E, Nakao, K, Takano-Yamamoto, T, Kasugai, S, Saito, M & Tsuji, T 2011, ‘Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy’, PLoS One, vol. 6 no. 7, p. 21531.
Prescott, R, Alsanea, R, Fayad, M, Johnson, B, Wenckus, C, Hao, J, Johns, S & George, A 2008, ‘In-vivo generation of dental pulp like tissue by using dental pulp stem cells, a collagen scaffold, and dentine matrix protein 1 after subcutaneous transplantation in mice’, Journal of Endodontics, vol. 34 no. 4, pp. 421-426.
Ravindran, S & George, A 2015, ‘Biomimetic extracellular matrix medicated somatic stem cell differentiation: applications in dental pulp tissue regeneration’, Frontiers in Physiology, vol. 6, p. 118.
Ravindran, S & George, A 2014, ‘Multifunctional ECM proteins in bone and teeth’, Experimental Cell Research, vol. 325 no. 2, pp. 148-154.
Ravindran, S, Zhang, Y, Huang, C & George, A 2014, ‘Odon-togenic induction of dental stem cells by extracellular matrix-inspired three-dimensional scaffold’, Tissue Engineering Part A, vol. 20 no. 1-2, pp. 92-102.
Rosa, V, Zhang, Z, Grande, R & Nor, J 2013, ‘Dental pulp tissue engineering in full-length human root canals’, Journal of Dental Research, vol. 92, pp. 970-975.
Sanjana, N & Fuller, S 2004, ‘A fast flexible ink-jet printing method for patterning dissociated neurons in culture’, Journal of Neuroscience Methods, vol. 136, pp. 151-163.
Thesleff, I &Tummers, M 2003, ‘Stem cells and tissue engineering: prospects for regenerating tissues in dental practice’, Medical Principles and Practice, vol. 12 no. 1, pp. 43-50.
Wang, Y, Zhao, Y, Jia, W, Yang, J & Ge, L 2013, ‘Preliminary study on dental pulp stem cell-mediated pulp regeneration in canine immature permanent teeth’, Journal of Endodontics, vol. 39 no. 2, pp. 195-201.
Yu, J, Wang, Y, Deng, Z, Tang, L, Li, Y, Shi, J & Jin, Y 2007, ‘Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells’, Journal of Cell Biology, vol. 99 no. 8, pp. 465-474.