Introduction
Diabetes mellitus is a metabolic disorder characterized by glucose intolerance. It is a systemic disease caused by an imbalance between insulin supply and insulin demand. Insulin is produced by the pancreas and normally maintains the balance between high and low blood glucose levels. In diabetes mellitus, either there is not enough insulin or the insulin that is produced is ineffective, resulting in high blood glucose levels. It also causes disturbances in protein and fat metabolism. These abnormalities are associated with micro-and macrovascular and neuropathic changes (Carol, 2005; Freshwater & Bishop 2004).
There are two types of diabetes mellitus namely insulin-dependent (IDDM/Type I) and non-insulin dependent (NIDDM/Type II). Research has shown that obesity is one of the most important determinants for the development of NIDDM. It is estimated that 80% of all clients with NIDDM are obese (20% over ideal body weight). Increasing age may be a risk because the pancreas becomes more sluggish with age in clients who are already predisposed to diabetes (Black, & Matassarin, 1993). NIDDM is due to the insensitivity of the glucose-sensing mechanism of the beta cells, and in obese patients, there is a decrease in the number of insulin receptors on the cell membrane of muscle and fat cells. Obese people do secrete a lot of insulin but it is ineffective because of the decreased number of receptors (Clark, 2003). Its development is consistent with all the pathophysiologic changes seen in long-term obesity, with the pancreas failing to compensate for insulin due to problems of the receptors (Black, & Matassarin, 1995). Type II diabetes has no symptoms in the early stages. Later symptoms include polyuria, polydipsia, and polyphagia followed by weight loss, weakness, and fatigue; hyperglycemia leading to glucosuria, osmotic dieresis, and the loss of water and electrolytes; excess ketogenesis; slow healing of cuts, blurred vision, cramps in the legs, feet, and finger itching. It is worth noting that type II diabetes is common in people aged 40 years and above but may be seen in obese children (Carol, 2005; Sally, & Rosamund, 2004).
Community Diagnosis
Community Profile
In this project, I will be discussing the state of Kuwait in relation to health issues with a special interest in Diabetes type II (NIDDM). This is a state that has in the recent past reported increasing cases of diabetes type II.
Geographical Profile: the state of Kuwait is located on the western side of the Persian Gulf and it borders Iraq to the north and west and Saudi Arabia to the south. It has a coastline totaling 499 Km with its territory largely made up of desert. It experiences hot summers and short cool winters with average temperatures of 38c in august and 13C in January. Rainfall is hardly experienced with 26 rainy days annually. The state of Kuwait covers an area of 17,820 square kilometers and a population density of approximately 126 per square meter. The state is located in a desert region and therefore experiences tropical weather. According to a censure carried out in 2001, the total population was found to be 2.25 million with a life expectancy of 73.8 years (the State of Kuwait, 2009). Political Profile: Kuwait is a sovereign state and has its own constitution under the presidency of his highness the Emir of the country. The state is a national assembly composed of fifty members who are elected through a democratic election which are held every four years. The national assembly is also known as the ‘state council’ and is charged with the responsibility of legislation. Laws and policies on health are passed and amended in the national assembly and therefore political leaders have a great impact on the health care system in Kuwait (the State of Kuwait, 2009). Socio-Economic Profile: Kuwait is predominantly a Muslim state with Islam being the official religion of the state. Other religions do exist such as Christianity which is practiced largely by expatriates. Non-Muslims enjoy the freedom of practicing their religion without interference from any quarter. Kuwait is endowed with oil resources and natural gas which have made it one of the highest per capita incomes in the world. This led to a crease in its population by 25%, including immigrants for the last six years. There is rigid segregation and extreme nationalism in that more than half of the population is non-Kuwaiti. The youth (under 20 years) forms the majority of the population as they comprise 60%. In the recent past, the Kuwaiti government embarked on a strategic plan aimed at distributing wealth amongst its citizens with a substantial amount being spent on the health care system, education, and public works. The ministry of health accounts for approximately 20% of the total budget. The Kuwaiti education system represents an integration of development with the provision of medical clinics for students. Available data shows that there has been a steady increase in the number of enrollment in schools and colleges at all levels with the increasing number of teachers, lecturers, and tutors. The government of Kuwait has been ensuring the provision of social care services in all fields in order to provide and improve the living standards of its citizens in order to create a sense of social togetherness and cohesiveness. This has in turn protected them against instability and created a sense of belonging (the State of Kuwait, 2009).
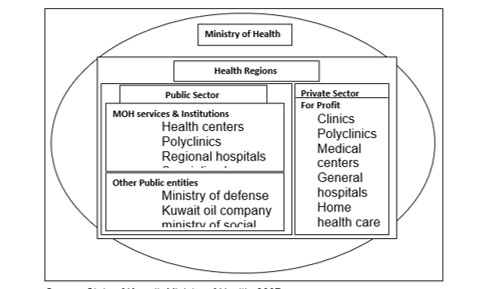
Health Care System: The state of Kuwait has a health care system based on the provision of primary health care with the centers being at the lowest level of the health care system. This type of system allows all sectors of the population to be reached. Combinations of clinics offer primary care and specialty clinics (MOH, 2007). Public Health care system
Central MOH: The ministry of health is charged with the responsibility of the planning, financial, resource allocation, regulation, monitoring, and evaluation in addition to health care service delivery. Health Regions: Kuwait is divided into 6 health regions namely Capital, Hawal, Ahmadj, Jahr, Farwanja and Suabah. These regions offer health care services as per the ministry’s guidelines. The main responsibilities of the regions are provision of health care services to its residents through putting into action the MOH’s action plan; provision of health care at different levels and types; ensuring training of different medical staffs; and ensuring a computerized database for health information. The health regions are headed by the director of health. The regions have general hospitals and several primary health centers and specialty clinics. Primary health care is delivered to the consumers through the health centers with family clinics, MCH & FP clinics, Diabetic Clinics, dental and preventive care clinics. The six regional hospitals provide secondary health care through a network of national specialized hospitals and clinics (MOH, 2007).
Private Health Care System:
Modern, for-profit: The private sector focuses on curative services rather than preventive services. That is not much information on the number of private clinics.
Modern, not-for-profit: these basically include hospitals owned and managed by oil companies and they include Ahmadi, Texaco, and Kuwait National Petroleum Company (KNPC) hospitals. By the year 2001, the number of health institutions and units was 97 hospitals and units, physicians were 3000 with a bed capacity of 5,000 (MOH, 2007).
From the above profile, we can deduce that the health care system is so widely and equally distributed across the country and therefore it is possible that each and every individual can access health care within a radius of 5 KM. the health care system focuses on primary health care which is generally at the community level meaning that the community members even at the lowest level and remote areas can still benefit from health care services being provided for by the government. According to a report by Al-Hooti (2008), diabetes and other related nutritional diseases were found to be the leading causes of death in government hospitals. Another study by the Kuwait Foundation for the Advancement of Sciences (KFAS) found out that type II diabetes was the fastest growing in Kuwait. KFAS, therefore, financed the development of the Dasman Center for Research and Treatment of Diabetes in 2001. This center dedicates 30% of its effort to the education and training of the community and health professionals. A number of regional diabetic clinics have been established to help curb diabetes. This shows the seriousness with which the government working to tackle diabetes (Al-Hooti, 2008).
Community Assessment Plan
The process of community development is one of the fundamental roles of nurses who work in the community for purpose of helping the communities to realize their weaknesses and how to overcome these weaknesses. As mentioned earlier, diabetes type II is a problem in Kuwait and therefore needs to be handled at all levels. Community assessment is a systematic process in which the nurse together with the members of the community determines the health problems & needs of the community & develops plans of action and implements those plans. The process is outlined below (Zerwekh, 2003; Holloway, & Wheeler, 2002; Grol 2000). Exploration: During the initial stages of planning the community assessment, the nurse carries out a community inventory; this is described as a step of mapping out the community with the purpose of obtaining baseline information that helps plan for the rest of the assessment process. The nurse meets government officials and local leaders to brief them on the planned assessment; this will allow the nurse to have clearance from the relevant people. This also enables the nurse to have general knowledge of the terrain, roads/paths, and the kind of people he/she will encounter (Mwangi, 2008). Planning for Assessment exercise: the following will be the objective of the assessment: to determine the disease (diabetes type II) burden on the community; to determine the most affected by diabetes type II in terms of age, sex, socioeconomic status; to determine the etiology and associated factors of diabetes type II; to determine the predisposing factors; to determine knowledge and attitudes of the community on diabetes type II and to determine what is currently being done by health care system, and community to curb diabetes type II. An assessment tool (refer to appendix) will be used to collect the required information from the community members and any other relevant person (ICN, 1987 & 1997).
Recruitment and training of assistants: the nurse in charge of the assessment trains a few locals who will assist in data collection using the assessment tool. The trainees will also be involved interpreting of questions in the tool to the community members and therefore they need thorough training. Pretesting and reworking of the tool: the nurse takes the initiative of pretesting the tool using people with similar characteristics as those of the community where the assessment will be done. This helps to detect faults and shortcomings after which corrections are made.
Execution of the Assessment: this stage involves actually going to the community and engaging the community in discussions and giving them the assessment tools so that they can feel it with relevant information. The nurse and her/his assistants help the community members fill the assessment tool through interpreting questions that one does not understand. The collected information is coded, grouped, and analyzed. During the process of assessment, some of the people who will be consulted apart from the community leaders and government representatives are community health workers working with the community. This is a group of people who are knowledgeable and understand better the disease burden and process within the community. The health centers within the community will be a major stakeholders in the assessment process. The target group for the assessment will be adults aged 40 years and above and obese young adults as they are at risk group. Type II diabetes is known to affect these two groups and therefore the need to focus on them.
Critical Analysis of the Findings and Recommendations
From the collected data, it is evident that poor nutrition and lack of exercise are the major causes of type II diabetes in Kuwait as most of the individuals assessed were overweight. This is confirmed by a study carried out by Al-Hooti, (2008) titled “food consumption pattern for the population of the State of Kuwait based on food balance sheets” this study concluded that; “the food supply in Kuwait provided an excess of RDAs at a rate of 1.19 times of energy, 2.1 times of protein, 2.59 times of vitamin A, 1.37 times of thiamine, 1.39 times of riboflavin, 1.41 times of niacin, 2.52 times of vitamin C, 1.56 times of iron, and 1.10 times of calcium daily requirements”. This excess provision of nutrients is likely to lead to overweight putting the individual at risk of type II diabetes. The assessment also revealed that the community members have knowledge deficits concerning diabetes as they could not differentiate between the different types of diabetes and predisposing factors, causes, treatment, and management. The lack of knowledge is reflected in the poor eating habits and lack of exercise leading to overweight. Diabetes is also a burden to this community in terms of productivity and financial constraints in that the affected individuals spent more time in health care facilities instead of going to work hence spending more money on treatment with the government using a lot of its revenues directly or indirectly in the management of diabetes. The assessment has also revealed that a lot is being done in terms of prevention and treatment of diabetes although these interventions are not effective as they do not involve the community members hence no sense of belonging. The community is only viewed as consumers of the health care services instead of being viewed as major stakeholders in the management and prevention of type II diabetes and also as managers of their own health.
From the above, it is clear that the following needs to be done: involving the community in issues concerning their health; health education and promotion programs on diabetes (etiology, risk factors, signs and symptoms, treatment and management, complications and prognosis); rehabilitation of affected individuals to avoid readmissions; establishment of Diabetic clubs where the affected individuals can be meeting occasionally to share their feelings, new ideas and personal experiences. Health Education and Promotion is one of the major interventions that will be carried out within the community. As already mentioned, knowledge deficit is a lethal weapon against the prevention, management, and control of type II diabetes. It is therefore the duty of the nurse to organize awareness campaigns amongst the community with the purpose of educating them on all relevant issues on diabetes (type II). Health education/promotion empowers an individual with the much-needed and relevant information that can be of great assistance in the management of his/her health and other related issues (Thompson, 2004). The nurse can carry out this activity with the help of the local health professionals working within the community and even train some community members who will be educating their colleagues; this creates a sense of belonging and ownership among the community members in that they will participate in the health education/promotion activities as their own. This empowers the community and the information stays with them even years after the time of carrying out the assessment.
Evaluation
Just as in the nursing process, evaluations help in checking if the assessment was a success and whether there has been any positive impact, and if interventions put in place had desired results. The evaluation also helps in knowing if the set goals and objectives were met, determining the success or failure of the problem, and to put the corrective measures into place (Elaine, 2005; Jorsen, 1999). This assessment was a success as the objectives were met and findings implemented.
From this assessment, the following have been brought out as some of the roles of the nurse during community assessment: management role-planning, organizing, controlling, staffing, and directing; Technical adviser -Sharing technical information with individuals, and communities; Assessor/ identifier – assessing the health status of the community. Identify existing & potential health problems; Health promoter – Sharing of prime health messages to promote the health of individuals, families, and the communities; Evaluator – Determines performance and outcome of community healthy activities; Trainer – He/she has the responsibility to train the community members on issues related to diabetes (Field, & Lohr, 2000, ICN, 2007).
References
Al-Hooti, S. N., (2008). Food consumption pattern for the population of the State of Kuwait based on food balance sheets. Safat, Kuwait: Kuwait Institute for Scientific Research.
Black, M. B. & Matassarin, E. J., (1993). Medical surgical nursing: A psychophysiologic approach. 4th Ed. London: W. B. Saunders Company.
Black, M. B., & Matassarin, E. J., (1995). Adult nursing: A psychophysiologic approach. London: W. B. Saunders Company.
Carol, T., (2005). Fundamentals of nursinmg: The art and science of nursing care. Philadelphia: Williams and Williams.
Clark, M. J., (2003). Community Health Nursing; Caring for populations. 4th Edition. New Jersey: Pearson Education Inc.
Elaine, M. M., (2005). Health Bulletin – Promoting Healthy Behaviour. Washington DC: Population Reference Bureau.
Field, M. J., & Lohr, K. N., (2000). Clinical practice guidelines: directions for a new program. Washington, DC: National Academy Press.
Freshwater Dawn., & Bishop Veronica., (2004). Nursing Research in Context; Appreciation, Application and Professional Development. New York: Palgrave Macmillan.
Grol R. (2000). National standard setting for quality of care in general practice: attitudes of general practitioners and response to a set of standards. Br J Gen Pract ; 40:361–4.
Holloway, I., & Wheeler, S., (2002). Qualitative Research in Nursing (2nd Ed.) Oxford: Blackwell Publishing
International Council of Nurses (2007). Management of Nursing and Healthcare Services.
International Council of Nurses, (1997). Nursing Research: Building International research Agenda. Report of the Expert Committee on Nursing Research. Geneva: ICN.
International Council of Nurses., (1987). Blueprint for ICN Programme 1988-1993 toward more effective participation in Health Policy Making and Healthcare Delivery. Auckland, New Zealand.
Jorsen, D. (1999). History and Trends of Professional Nursing. Washington DC: Mosby Company.
Ministry of Health, State of Kuwait, (2007). Ministry of health; health care service provision and delivery. Kuwait: Government Printer.
Mwangi, K. N., (2008). The Process of Community Diagnosis. Naorbi, Kenya: Upendo Publishers.
Sally, K., & Rosamund B., (2004). Primary Health Care Research and development. London: Arnold, Hodder Headline Group.
State of Kuwait, (2009). General information about Kuwait.
Thompson, D., (2004). Nursing fundamentals: Caring and clinical decision making. New York: Rick Daniels.
Zerwekh, J., (2003). Nursing Today: Transition and Trends. Philadelphia: W. B. Saunders Company.
Appendix: Tool of Data Collection


