Introduction
The knowledge of the chemical structures of chemical compounds and the relationship with the biological or physiological activity they can elicit is very important in the development of drugs. This relationship can be used to assess the compound’s toxicity. The study of the structure-activity relationship is critical to biochemistry and pharmacology since when we discover what molecules can elicit some biological reaction, then that can be harnessed for the betterment of life. However, this is very complex but somehow chemists and pharmacologists intuitively recognize it. In order to offer the solution, it’s important to understand the scope of this issue. Quantitatively, the study of the structure-activity relationship connects the structure to the descriptor. Several descriptors assist in defining the problem; hydrophobicity, steric effects, typology and electric properties.
History of QSAR
The GSAR technology exploration began as early as the 19th century; the early observation was about the effect of alcohol had toxicity to animals and the effect was higher with the decrease in alcohol solubility (Bevan, para 1). This was a very critical observation that led to the discovery that the toxicity of such organic chemicals (compounds with C and H) was highly dependent on lipophilicity. Meyer Hans independently made this discovery in the 1890s (Bevan, para 1).
Free energy Correlations were advanced by Louis Hammett. He was able to correlate the electronic characteristics of organic bases and acids with their reactivity and the subsequent k – constants (equilibrium constants) (Bevan, para 3). Benzoic acid for instance;
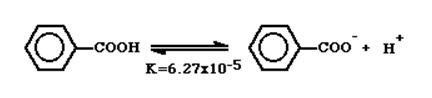
The addition of constituents to the benzene ring produced an orderly and quantitative impact on the dissociation constant. A nitro group (N2O) added to the meta-position increased the dissociation constant as the nitro group withdraws electrons hence making steady the negative charge (Bevan, para 4).
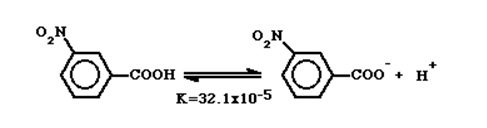
In the para position, the nitro group (N2O) shows greater energy of dissociation implying greater withdrawal of electrons (Bevan, para 5).
The trend is the same when other constituents are added. Ethyl added to the para position indicates a lower dissociation constant demonstrating that it was an electron donor (Bevan, para 6).
Introduction to Drug Design
Drug development in research is a very expensive undertaking due to the cost that the process entails. The process entails identifying the targets that are to be bound by the drug. These targets are usually referred to as receptors and are in most cases proteins (Borman, 21). The classical methods under this technology usually describe the structure activity in relation to the physico-chemical characteristics as well as the steric characteristics (Bevan, para 7). They include the Hansch analyses, extra-thermal models and also some of the structural components of like free Wilson analyses. Basically, the three-dimensional models are very easy to study and understand. They are also widely studied (Borman, 21). These structures take into consideration the structure of a compound in three-dimensional and express the binding modes that are utilized by the protein ligands (Borman, 21).
The QSAR models are founded on the assumptions that the linear additive contribution various structure characteristics or features a certain compound to the biological activity, so long as other non-linear dependencies of binding or transporting on particular physico-chemical properties do not exist (Borman, 21). This simple assumption has been valid by several committed researches, for instance, the de novo drug designing scoring function, as well as several analyses from Hansch and Free Wilson, approaches.
It’s imperative to note that the QSAR identifies that these molecules (organic elements, receptor proteins, etc) are actually three-dimensional. Most important of the characteristics are the steric position (shapes), electronic aspects (electric charges) and the solubility (polar and non-polar).
There are several steps that are taken by QSAR; the first is to convert the molecular structure into a mathematical descriptor that covers all the major properties of the compound (molecules) appropriate to the activity or characteristic being described (Borman, 21). The second is to select the best descriptor from the numerous set of available and appropriate descriptors. The third is to map the compound (molecular) descriptor into the characteristics, the model-free is preferably used since there are no assumptions required to apply to the functional design of the structure. The relationships here are usually very complicated and not-known as they as also non-linear (Borman, 21). Fourth, is to validate the model to assess how predictive it can be and the manner in which it can generalize in relation to the compounds and elements, not in data being used to create the model.
Hansch Analysis
From the Hammett equation the indicates the linear relationships between the electric coefficient from the dissociation constant;
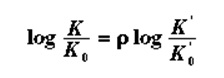
From the above equation, p represents the gradient of the slope while the sigma sign σ represents the abscissa values (Borman, 22).

This relationship is so important since it relates the proportionality constant of the equilibrium to the impact the substituents have at the equilibrium. δ describes the substituents and described the capacity to donate or withdraw electrons. Hammett equation is a free energy derivative. It, therefore, follows the equation of energy;
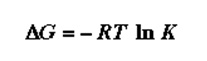
A QSAR was developed from the Hammet equation to show the ability of a drug to inhibit growth in relation to its ability to ionize. The Hammett equation did not show meaningful outcomes on close analysis. Muir and Hansch realized that there were some other factors that were very critical to this drug design. Therefore, the electronic effect alone could not cater to the biological effect (Borman, 22). Hansch realized the importance of solubility of the drugs at this instance. The following equation was derived lipophilicity as a factor that is important in determining the bioavailability of the drug in circulation and the target receptors. This was expressed in terms of the octanol-water partition coefficient. In some instances, the univariate correction between the structure and the biological activity was appropriate (Borman, 23). The equation for this was hence;
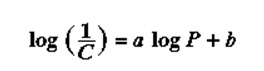
Where C represents the Molar concentration, which is able to elicit a standard reaction. For instance, the lethal dose of 50% (LD50) or the effective dose of 50% (ED50): the data was seen to improve when Hammett electronic characteristic and lipophilicity from Hansch were combined (Richon & Stanley, para 6). The resulting equation is;
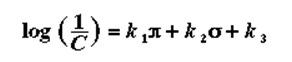
δ Represents Hammett substituent factor while the pi π sign described the sigma analogously as in
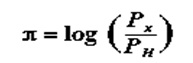
In some instances, however, the parabolic relations were seen to offer the best fit in describing hydrophobilicty and a (log P) **2 factors in the qualitative Structure relations. To account for the factor, an explanation given is that in the real activity of the drug, cell membranes have to be traversed for the drug to get to the target receptor. Drugs with high hydrophobicity get stuck in the membranes (Richon & Stanley, para 6).
Drug Design
Over the years, researchers have been developing and making trials for drugs based on the information obtained from the QSAR studies. The efficient computational was not accessible and the attempts to develop drugs were entirely statistical descriptions of the structure of the drug (Richon & Stanley, para 7). The basic example of drug design that adopted QSAR technology was the 1-(X-phenyl)-3,3-dialkyl triazenes series.
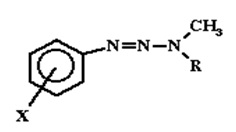
The chemical was critical since they were acting against tumors yet cancers were dreaded the most by that time. Modifying the structure would decrease the mutagenic activity of the cancers (Richon & Stanley, para 8). From the Ames tests, mutagenic activity was characterized and the following QSAR developed:
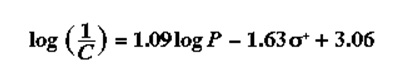
C describes the molar concentration that gave 30 reveretants 10*8 bacteria and this was attained under a resonance of [8,9] (Richon & Stanley, para 9). The example indicates that mutagenicity was affected by electron activity and lipophilicity. Following this development, several studies on mice regarding tumors have been conducted and drugs developed from this knowledge.
Conclusion
There are alternatives that have been advanced to take care of the combinational difficulty that is experienced by Hansch’s analyses. This is results from Free and Wilson’s ability to incorporate some specific constants of substituent whose physiologic effects were associated with the presence of the functional groups at specific points on the parent compound. The equation used is:
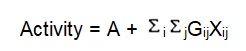
Where A = average biological action elicited.
Gij = the input of the functional group
i = the jth position
Xij = existence or absence of exact functional group at jth point.
There are several cases when biological activity cannot be determined accurately. This is because of a range of reasons, for instance when a certain system lacks sensitivity. Other techniques have been suggested to address this problem. Labeling the elements active, inactive or partially active is very important.
Works cited
Bevan, David. QSAR and Drug Design. Network Science Corporation. 2009. Web.
Borman, Stu. New QSAR Techniques Eyed for Environmental Assessments. Chem. Eng. News, 68: 20 – 24. 1990. Web.
Richon, Allen and Young, Stanley. An Introduction to QSAR Methodology. Network Science Corporation. 2009. Web.