Introduction
Antipsychotic drugs are classified as the group of drugs which are used to treat psychotics. Most commonly, these are the representations of schizophrenia, and sometimes the severe bipolar disorders. The aim of this paper is to give and explain the classification of the antipsychotic drugs belonging to different pharmacological and chemical classes. Originally, the chemical classes of the analyzed drugs, such as Chlorpromazine C17H19ClN2S, Thioxanthene C23H25F3N2OS, Haloperidol C21H23ClFNO2, Loxapine C18H18ClN3O, Risperidone (Risperdal) C23H27FN4O2, Olanzapine will be evaluated in accordance with various aspects and characteristics, such as structural analysis, acid-base characteristics, ionization processes, polarity, solubility, partition and the theoretical metabolic pathways.
Classification of Drugs. Chemical Classes and Characteristics
Originally, the main aim of these medications is to influence the chemistry of the brain, thus, the chemical features of these drugs are of the primary importance for medicine. Thus, the chemical classes of the analyzed drugs will be regarded first.
According to Bronsted-Lowry theory, (Adjepon-Yamah, Scott, Prescott, 2003) drugs behave in solutions as acids if they donate at least one proton such as: AH→ A- + H+. On the contrary, if the drug molecules have at least one lone pair of electrons that can bind and accept a proton, they are called bases such as: B: + H+ → BH+. In accordance with Hansen (2001) Amphoteric drugs have both acidic and basic functional groups that can donate and accept protons respectively. Some drug molecules exist without basic or acidic functional groups and therefore are classified as non-electrolytes.
All of the chemicals analyzed in the paper are subject to ionization as the tertiary amine group is generally ionized during the reactions with water, thus forming a quaternary amine salt with a positive charge in accordance with the equation:
R3N + H2O = R3NH+ + OH
In the light of this consideration, it should be emphasized that the drugs with the highest pKa value would form the strongest bases and more prone to ionization. These drugs are chemically basic, and originally this means that they will be ionized (BH+) in an acidic medium such as the stomach. (Reines, 2000) The absorption of the unionized components of the analyzed remedies will be represented in the corresponding parts of the paper
The absorption scheme
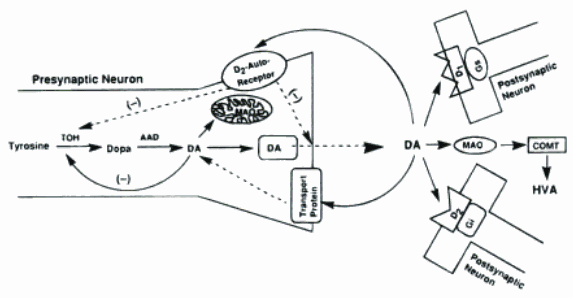
It is generally stated that tyrosine, which is generally the key component of the Antipsychotics drugs, is hydroxylated in a rate-limiting step by tyrosine hydroxylase (TOH) to form dihydroxylphenylalanine (DOPA), which is decarboxylated by L-aromatic amino acid decarboxylase (AAD) to form dopamine (DA). The DA is stored in vesi-released into the synaptic cleft may go on to stimulate postsynaptic D1 and D2 tyoe autoreceptors that modulate DA synthesis and resease. Cytoplasmic pools of DA may undergo metabolic deamination by monoamine oxidase (MAO), an enzyme bound to the outer membrance of mitochondria. (Foye, Lemke, Williams, 2007)
The pathways of the absorption of the drugs is represented on the scheme:
These pathways are general for all the antipsychotic drugs, thus, there is no necessity to give this information on every drug.
Drug Metabolism Factors
The biochemical modification of drugs is generally observed when the components are absorbed or react with the body fluids. As Leucht, Leucht and Hamann (2003) emphasizes: “Drug metabolism often converts lipophilic chemical compounds into more readily excreted polar products. Its rate is an important determinant of the duration and intensity of the pharmacological action of drugs.” The factors, which generally influence metabolism mainly depend on the liver’s activity and its reaction for the drug consumption. Rabbani and Greenough (2004, p. 156) emphasized the following: “If a drug is taken into the GI tract, where it enters hepatic circulation through the portal vein, it becomes well-metabolized and is said to show the first pass effect”.
Acid Base Characteristics
It should be stated that all the medications, regarded in the research paper are featured with the close to neutral acid-base characteristics. None of them is too acidic or basic except Rsperidone. Thus, the features are represented in the table:
Antipsychotics are mainly attributed to a functional group where the oxygen is flanked by two alkyl groups. These compounds can H-bond weakly to water, nevertheless, they are not polar to be water soluble. One of the most prevalent acid-base functional groups in drug molecules is the amine group. (Reines, 2000, p. 237)
Drug Review
Chlorpromazine
C17H19ClN2S
The Chemical structure of the molecule is represented on the following scheme:
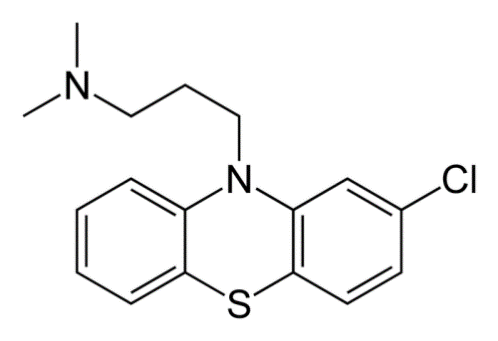
The molecular mass is 318.86 g/mol (freebase) and 355.33 g/mol (hydrochloride).
The chemical features of this remedy, intended for influencing the brain chemistry processes are meant to be mild enough for not violating the structure of neurons and strong enough for fighting the defections. Thus, as Reines (2000, p. 256) claims:
“The permanent positive charge on the distal nitrogen atom of the tricyclic side chain contribution to binding was estimated as >/=5.6 kcal.mol(-1) by comparison with the analogue with the cationic nitrogen atom of the quaternary replaced by an ether oxygen atom. A further major contribution to improving K(i) values and inhibition strength was the hydrophobic natures and structures of the N-benzyl substituents. The strongest inhibitor, the dimethylammonium derivative (K(i).12 microM), was approximately 2 orders of magnitude more inhibitory than the parent chlorpromazine.”
As for the matters of distribution, it should be emphasized that the distribution levels of orally administered Chlorpromazine are the following:
The optically active isomers rates are represented in the following table:
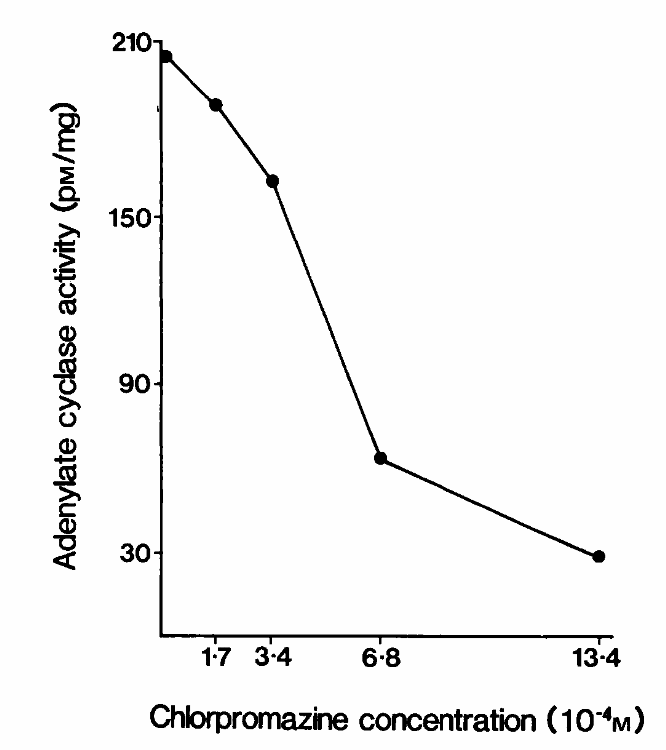
The scheme represents the Inhibition of Adenylate cyclase activity stimulated by LT-enterotoxin by increasing doses of chlorpromazine during incubation time of 45 min. (Hansen, 2001)
Chlorpromazine hydrochloride can interact with some other medicines. The list of these medicines is the following:
- Alcohol
- Barbiturates
- Certain anticonvulsants
- Certain SSRI antidepressants
- Narcotics or opiates, such as morphine or oxycodone
- Pindolol
- Propranolol
- Rifamycin antibiotics, including rifabutin, rifampin, and rifapentine
- Tricyclic antidepressants
Thioxanthene
C23H25F3N2OS
The thioxanthenes, as a class, is chemically close to phenothiazines. Nevertheless, instead of nitrogen at position 10 in the phenothiazines, thioxanthene entails a carbon atom on this position. Thus, the atomic structure is the following:
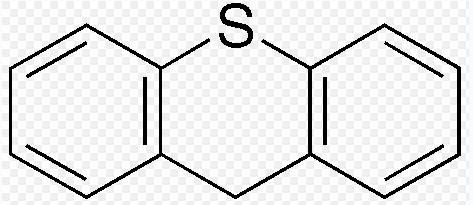
As Leucht, Wahlbeck and Hamann (2003, p. 410) emphasizes:
Thioxanthene is a molecule in which the oxygen atom in xanthene is replaced with a sulfur atom. Various thioxanthene derivatives are used in the treatment of schizophrenia and psychosis. The activity of these compounds is related to their ability to inhibition transmission at D2 receptors, in the brain.
As for the issues of intermolecular drug binding interactions, it should be stated that the standard formation (p = 0.1 MPa) of crystalline thioxanthene (117.4 ± 4.1 kJ·mol−1) was defined only by the means of the investigational standard molar energy of combustion in oxygen. Thus, the experiment presupposed the measuring by rotating-bomb calorimetry at T = 298.15 K (Rabbani, Greenough, Holmgren, 2004, p. 219)
The issues of alkylation are generally represented in the matrix of the folded structures, which are capable of displaying s substituent bound to a meso position in either the pseudo axial or the pseudo equatorial position. (Reines, 2000)
The rates of optically active isomers of the molecule are represented in the following table:
As for the matters of distribution, it should be emphasized that the distribution levels of orally administered Thioxanthene are the following:
Haloperidol
C21H23ClFNO2 Mol. mass 375.9 g/mol
It is a typical antipsychotic, consequently, the chemical features of this medication are close to neutral, as the reactions and effects should be as less harmful as possible. The pharmacological effect is close to phenothiazines.
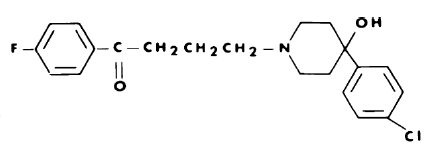
The optically active isomers of the haloperidol molecule are in some ways quite different from those of the corresponding racemic mixture. Originally these rates are represented in the following table:
The interaction with other drugs is not studied perfectly, nevertheless it is not recommended to consume with those which affect the heart rhythm as for the matters of distribution, it should be emphasized that the distribution levels of orally administered Haloperidol are the following:
Loxapine
C18H18ClN3O
Mol. Mass. 327.808 g/mol
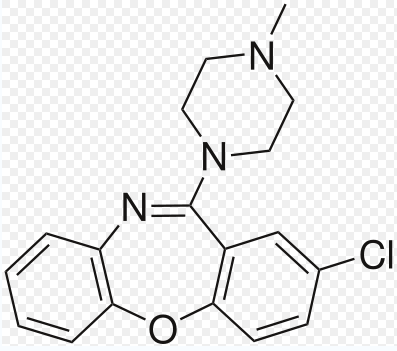
The basic pharmacological features are close to Haloperidol, nevertheless, its atomic structure is not stable as the structure of Haloperidol.
From the chemical point of view, it should be stated that Loxapine is generally metabolized by N-demethylation to amoxapine, a tricyclic antidepressant. Some components change the structure completely. Thus aluminum salts decrease the absorption of antipsychotics on the basis of Loxapine.
- If amphetamines are consumed with Loxapinem the psychotic symptoms may even enforce;
- The use of antipsychotics with an antihypertensive may produce additive hypotensive effects.
Loxapine interacts with the following medicines:
- Amantadine
- Apomorphine
- Bromocriptine
- Carbamazepine
- Levodopa-containing medications
- Lorazepam
- Pramipexole
- Ropinirole
Nevertheless, it should be stated that Loxapine may seriously lower the effectiveness of the remedies used for treating the Parkinson’s disease, including apomorphine. (World Health Organization, 2001)
The optically active isomers of the Loxapine molecule are represented in the following table:
Risperidone
C23H27FN4O2
Mol. mass 410.485 g/mol
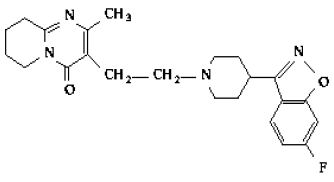
It is stated in Hansen (2001, p. 371):
Risperidone displays a novel mechanism of antagonism of the h5-HT7 receptor. Pretreatment of the cells with 5 or 20 nM risperidone, followed by removal of the drug from the media, renders the 5-HT7 receptors unresponsive to 10 μM 5-HT for at least 24 h. Thus, risperidone seems to be producing a rapid, long-lasting inactivation of the h5-HT7 receptor. Whole-cell radioligand binding studies indicate that risperidone interacts in an irreversible or pseudo-irreversible manner with the h5-HT7 receptor, thus producing the inactivation.
The list of the medicines, which Risperidone may interact with:
- Some anticonvulsants
- Cimetidine
- Clozapine
- Fluoxetine
- Paroxetine
- Ranitidine
- Rifamycin antibiotics.
The rates of optically active isomers of the Risperidone molecule are represented in the following table:
Olanzapine
C17H20N4S
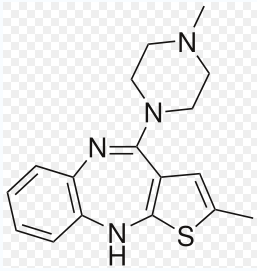
The list of the medicines, which Olanzapine may interact with:
- Alcohol
- Carbamazepine
- Ciprofloxacin
- Fluvoxamine
- Ketoconazole
- Mexiletine
- Norfloxacin
- Ofloxacin
The optically active isomers of the molecule possess useful pharmacological and therapeutic properties as anti-emetics and anti-cataleptics in some ways quite different from those of the corresponding racemic mixture. Originally the rates are represented in the following table:
As for the matters of distribution, it should be emphasized that the distribution levels of orally administered Olanzapine are the following:
Reference List
Adjepon-Yamah, K.K., Scott, D.B. & Prescott, L.F. (2003). “Impaired absorption and metabolism of oral lignocaine in patients undergoing laparoscopy” Br. J. Anesth., 45, 143-147.
Foye, W. O., Lemke, T.L., Williams, D.A. (2007) “Foye’s principles of medicinal chemistry” Lippincott Williams & Wilkins
Hansen E.H. (2001) “How widely do women and men differ in their use of psychotropic drugs?” J Soc Adm Pharm; 6: 165-83.
Leucht S, Wahlbeck K, Hamann J, et al. (2003) “New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis” Lancet; 361: 1581-9
Rabbani G H, Greenough W B, Holmgren J, Kirkwood B (2004) “Controlled trial of chlorpromazine as antisecretory agent in patients with cholera hydrated intravenously” British Medical Journal284: 1361-1364.
Reines, Brandon P (2000). “The Relationship between Laboratory and Clinical Studies in Psychopharmacologic Discovery”. Perspectives on Medical Research (Medical Research Modernization Society) 2.
World Health Organization. (2001) “Guidelines for ATC (Anatomical Therapeutic Classification)” Oslo: World Health Organisation Collaborating Centre for Drug Statistics Methodology,.