Introduction
Atmospheric chemistry is the field in science that deals with the atmosphere and the chemical reactions occurring in it. Earth’s atmosphere that forms the immediate cover is composed of oxygen, nitrogen, carbon dioxide, methane, and other gases. The chemical reactions that happen in the troposphere making new and different chemicals have a direct impact on human beings and all other life forms on the earth. The troposphere is the first atmospheric layer and is about 7 km at the poles and 17 km at the equator. The stratosphere is the next layer of the atmosphere and is from that 7 – 17 km range to about 30 km above the earth’s surface (Wikipedia n. pag, 2007a). This layer is rich in ozone and forms a proactive layer from the sun’s ultraviolet rays. The stratosphere is followed by the mesosphere, the heterosphere, and the exosphere.
Chemistry of the atmosphere
As mentioned before the earth is surrounded by a blanket of gases. This blanket of gases traps energy in the atmosphere, in a similar way a man-made greenhouse does. The earth’s natural greenhouse results in a build-up of energy, and the overall warming of the atmosphere. This is called the greenhouse effect and is a natural process because of which life on earth is possible. Without this, naturally occurring greenhouse gases such as water vapor, carbon dioxide, methane, and nitrous oxide, the Earth’s surface temperature would have been very cool, and live on it could have been impossible for all of us (Earth Science n. pag). Because of the increase of greenhouse gases due to man-made activities, the enhanced effect which is caused is increasing the earth’s temperature or in other words, global warming is occurring. Besides, greenhouse gases such as CFCs are responsible for depleting the ozone layer. The ozone layer is being damaged mainly by certain industrial chemicals, vehicular pollution, and also due to ozone-depleting refrigerants, halons, and methyl bromide (ESS n. pag, 2000).
A complex series of reactions occurring in the atmosphere results in the chemistry of the atmosphere. The presence of the sun’s UV rays makes these reactions more and more complex. In the troposphere, atmospheric chemistry forms ozone (a pollutant) and many highly reactive intermediates and molecules including acids, peroxides, and other undesirable products that can result in serious problems for human health and the environment. However, in contrast, stratospheric chemistry leads to the destruction of natural stratospheric ozone, our protective shield from short ultraviolet radiation at the earth’s surface (Calvert 1-12).
Oxygen cycle
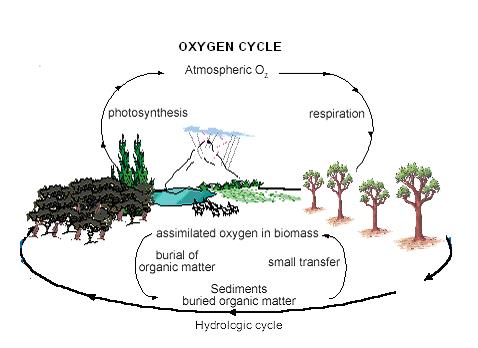
The oxygen cycle is the biogeochemical cycle that describes the movement of oxygen within and between its three main reservoirs: the atmosphere, the biosphere, and the lithosphere. Oxygen is mainly obtained as a by-product of photosynthesis. Since vast amounts of oxygen are present in the atmosphere, it is estimated that even if all photosynthesis were to cease it would still take about 2.5 million years to strip out more or less all oxygen.
Much of the discussion on the oxygen cycle has focused on the question of the origin of atmospheric oxygen and its variations over geologic time. Since both photosynthesis and respiration are cyclic processes, both involving release and utilization of oxygen there is a balance established. Through the process of photosynthesis, carbon dioxide and water is broken down to convert into sugar or glucose. As a by-product of this reaction oxygen is released into the atmosphere. The following is the reaction that occurs:
6CO2 + 6H2O + energy → C6H12O6 + 6O2
Photolysis is another source of atmospheric oxygen. In this chemical reaction, high-energy ultraviolet radiation from the sun breaks down atmospheric water and nitrite into component atoms hydrogen and nitrogen. These components escape into space leaving oxygen in the atmosphere. The following is the reaction that occurs:
2H2O + energy → 4H + O2
2N2O + energy → 4N + O2
Respiration and death and decay of organisms are the two main ways in which oxygen is lost from the atmosphere. In the decay mechanism, animal life and bacteria consume oxygen and release carbon dioxide. In addition, several other reactions are occurring in the atmosphere involving oxygen. Figure 1 shows the complete oxygen cycle in detail.
Natural pollution
Pollution is a major concern for the global community. Though man-made pollution and poor air quality are a major environmental concern due to industrial and vehicular pollution, there are many natural sources of pollution such as volcanoes, forest fires, death and decay, and others. They release probably much greater pollutants than their man-made counterparts.
Sulfur dioxide is a major pollutant that is released from volcanoes, biological decay, and forest fires. Oxides of nitrogen are released from volcanoes, oceans, biological decay, and lightning strikes. It is estimated that a range between 20 million and 90 million tonnes per year nitrogen oxides is released from natural sources when compared to around 24 million tonnes from man-made activities worldwide.
Ozone is yet another secondary photochemical pollutant formed as a result of chemical reactions taking place due to the presence of sunlight. About 10 to 15% of low-level ozone, however, is transported from the stratosphere, where it is created by the action of ultraviolet radiation on oxygen. In addition, particulates are another pollutant. Natural sources of particulate matter include volcanoes and dust storms. But man-made sources of particulate matter are higher when compared to natural sources.
Pollutants are also released by plants and trees. The most important of it is that green plants through evapotranspiration release a huge amount of carbon dioxide. Volatile organic compounds such as isoprene are naturally produced by plants and trees. These are believed to be a more significant trigger for asthma than man-made irritants. Pollen is yet another pollutant that occurs in the air all around the year. In addition, natural pollutants found indoors include dust-mite, mold spores, and radon gas.
It has become increasingly evident that Air pollution, in general, is affecting health and the environment. Though we all know for a quality life healthy environment are essential, it becomes even more important to preserve the environment for the future generation. Man-made sources of air pollution include deforestation, burning of fossil fuels, emissions from vehicles, industries, and agricultural activities. As a result of air pollution, there is an increase in global warming, ozone depletion, acid rains, smog, and other serious problems.
Ozone occurs naturally in the stratosphere. Even though it forms a small component of the atmosphere, it plays a vital role in limiting the amount of harmful solar ultraviolet radiation that reaches the earth’s surface. The ozone in the stratosphere is different from ground-level ozone. When ozone in the ground level increases it is an air pollutant and contributes to the smog over large cities. The formation of ground-level ozone occurs as a result of a chemical reaction between many forms of pollutants released from both natural and man-made sources and sunlight. Two main groups of chemical pollutants are involved: nitrogen oxides, and volatile organic compounds (VOC).
Ozone depletion in the upper atmosphere is the result of man-made chemicals, such as chlorofluorocarbons (CFCs) and halons. There are several treaties signed by many countries to reduce the concentration of these chemicals in the atmosphere. For instance, the Montreal Protocol, signed in 1987 and subsequently strengthened, has led to the phase out of most CFC and halon use. But regrettably, CFCs and halons remain for a long time in the atmosphere. Besides scientists in the Global Atmospheric Change Program are tracking changes to atmospheric concentrations of ozone-depleting chemicals and contributing to international efforts to assess and minimize ozone damage.
The main reason for acid rains to occur is the release of the gases such as sulfur dioxide and nitrous oxides. Besides the natural causes like volcanoes, the main sources of nitrous oxide emissions are vehicles and fossil fuel combustion. Sulfur dioxide reacts with water vapor and sunlight to form sulphuric acid. Similarly, nitrous oxides form nitric acid in the atmosphere. There are several harmful effects of acid rain. It can increase the acidity of lakes, and other water bodies and can cause the death of aquatic life. These can even be so harmful that they can erode buildings and monuments.
Smog mainly occurs in urban situations due to the high amount of pollutants in the atmosphere. It is a chemical mixture of gases that forms a brownish-yellow haze. The main components of smog include ground-level ozone, sulfur dioxide, oxides of nitrogen, volatile organic compounds, acidic aerosols and gases, and particulate matter. These gases are the result of the reaction between certain airborne pollutants and strong sunlight. Hence smog is linked with the amount of sunlight present and high temperatures and hence it is seasonal. Smog is common in the summer months when there is the most sunlight and temperatures are the highest. Smog is a threat to the animal, plant, and human life.
There is a worldwide concern about atmospheric pollution. There are several steps taken by governments to reduce the load of pollutants. However, every individual must take charge of this issue and take corrective steps to prevent pollution and safeguard the environment from further contamination. This will not only help to prevent human health problems but also every life on the planet earth.
References
Natural Air Pollution. 2007. Web.
Calvert , J. G, The chemistry of the atmosphere and its perturbations through human activities, Pure & Appl. Chern., (1997) Vol. 69, No. 1, pp. 1-12.
Collins, J. Acid Rain, 2001, Enviro Facts. 2007. Web.
CSIRO, Ozone depletion (2006) University of Cambridge, 2007. Web.
Earth Science, The Earth’s Atmosphere, Moorland School, 2007. Web.
ESS Earth’s Protection Shield is Being Destroyed – Ozone Depletion and Global Warming (2000) Environmental Support Solutions, 2007. Web.
Earth’s atmosphere. Wikimedia Foundation, 2007. Web.
Oxygen cycle. Wikimedia Foundation, 2007. Web.