Dear reader! In writing you this e-mail, I wish to attract your attention to the state of environmental protection in the petroleum industry. As it stands, it is one of the industries that define human society by providing fuel and materials for its continued existence. At the same time, it threatens nature and creates many long-term issues related to pollution of air, soil, water, the weakening of the ozone layer, and the facilitation of the greenhouse gas effect. However, these issues can be mitigated through technology
Introduction
The petroleum industry is one of the major energy producers in the world. According to Doric and Dimovski (119), petroleum power plants produce more than 40% of humanity’s entire energy pool. At the same time, the petroleum industry is one of the most significant environmental pollutants in the world. Almost every aspect of its existence poses a danger to the surrounding environment. Oil spills cause harm to animals, plants, water, and soil. Flares release smoke and chemicals into the air, as shown in Fig. 1 (Gates). Making benzene and other high-octane fuels from crude oil involve many environmentally unfriendly processes. Some of them include catalytic residue processing, delayed coking methods, and HF isobutene alkylation. Also, fuel refinery plants require plenty of water and oxygen to function.

These facilities are resource-intensive and potentially dangerous to life around them. According to the Environmental Protection Agency (EPA), the U.S. oil refining industry releases over 22000 tons of pollutants into the air every year. Actual numbers may be even higher due to widespread underreporting. The purpose of this paper is to examine the construction of technologically advanced refineries as a means of reducing pollution and improving the economic and environmental feasibility of the enterprise.
Solution: Environmentally Clean Fuel Refineries
Due to various regulations imposed upon the petroleum industry, many companies see the construction of new petroleum refineries as unprofitable. Instead, the companies upgrade the existing plants, which use environmentally unfriendly processes at the core of their operations. These plants pass the current regulations on safety and emissions, but just barely. To improve efficiency and eco-friendliness of the industry, it is necessary to implement new technological processes (Han et al. 292). Chen (17) identifies four conditions essential for a clean fuel refinery:
- Preventing heavy metals from entering the feed streams for the catalytic processing units.
- Removing heavy metals from feed streams using thermal processes.
- Converting coke into simple elements and chemicals (H2, CO, H2S, NH3, and metal oxides).
- Removing sulfur and nitrogen impurities from the feed stream by using advanced hydrogenation catalysts.
According to Sardameli (103), one of the potential solutions to satisfy all four criteria is a light olefin-based refinery plant. Light olefins are some of the primary raw materials used in the petrochemical industry. The new refinery will utilize thermal processes to eliminate solid catalyst waste, thus resulting in cleaner and more efficient production. An example of such a plant can be seen in Fig. 2 (SCG Chemicals).

New technologies, as proposed by Chen (17) differ from the existing practices in several ways. Fluid catalytic cracking (FCC) remains the primary fuel production process. However, the combustibles derived from it are light olefins, electricity, gasoline, and distillate. The bottom fraction, which is the most contaminated by heavy materials, is recycled and utilized as fuel for the hydrotreater. Increasing the hydrogen content of the FCC feed to match the carbon mole ratio should facilitate olefin production. The process of deep hydrotreating would satisfy the 1:1 H2/C balance, minimize the production of toxic waste, and reduce the plant’s gas solvent disposal (Chen 18). In addition, it requires fewer catalysts to facilitate the FCC process, thus reducing the production of solid waste and catalyst consumption. As it is possible to see, olefin plants provide a sustainable and ecologically-friendly solution to oil-refinery-related pollution.
Discussion
Olefin-based production has several advantages over the standard hydrocracking processes. According to Salkuyeh and Adams (489), olefin production can be facilitated using polygeneration processes, which involve the use of petroleum coke and natural gas. This process allows for zero carbon emissions while using pet coke and shale gas to produce methanol, DME, FT liquids, electricity, and olefins. The use of thermal processes helps remove nickel and vanadium from the feed streams, while hydrotreating significantly reduces the production of toxic emissions, catalyst consumption, and solid waste production. Salkuyeh and Adams (486) also propose four models of operational optimization to make olefin-based production economically viable. Due to low resource costs, consumption efficiency, and the multitude of produced products, the polygeneration process using shale gas and pet coke is financially feasible.
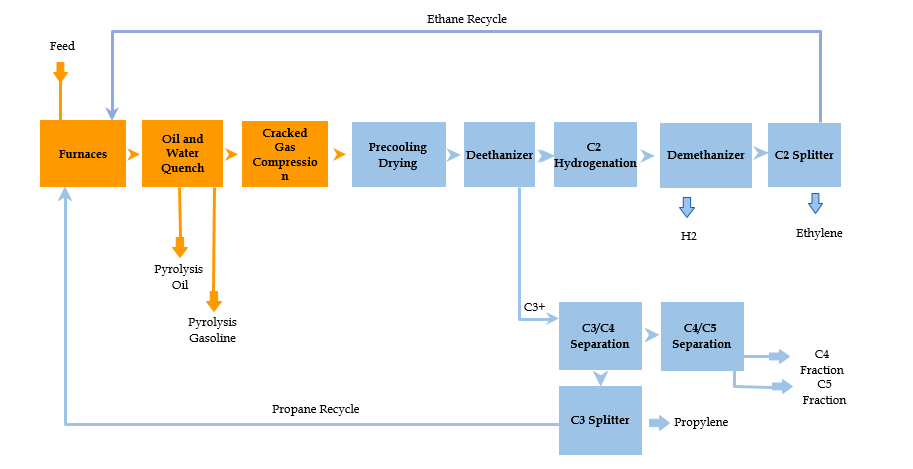
Amghizar et al. (171), however, claim that the availability of cheap propane, ethane, and methane are the primary determinants of the economic viability of the proposed solution. Shale gas and shale oil depositories provide the industry with these core elements. The economic viability of the proposed solution depends on how much it would cost to extract and transport these materials to the refineries. In order to maintain economic efficiency, the plants would need to process at least 120,000 bbl. A day. Steam cracking, as seen in Fig. 3, is the primary process currently in use for olefin production. It produces a wide range of air pollutants into the atmosphere. To achieve a better environmental impact, new oil refinery plants must forego steam cracking in favor of other methods of olefin production.
Works Cited
Amghizar, Ismael, et al. “New Trends in Olefin Production.” Engineering, vol. 3, no. 2, 2017, pp. 171-178.
Chen, Nai Y. “An Environmentally-Friendly Oil Industry.” Chemical Innovation, vol. 31, no. 4, 2001, pp. 10-21.
Doric, Barbara, and Vlado Dimovski. “Managing Petroleum Sector Performance – A Sustainable Administrative Design.” Economic Research – Ekonomska Istrazivanja, vol. 31, no. 1, 2018, pp. 119-139.
Gates, Sarah. Tesoro Flaring. 2017. Azavea, Web.
Han, Jeongwoo, et al. “A Comparative Assessment of Resource Efficiency in Petroleum Refining.” Fuel, vol. 157, no. 1, 2016, pp. 292-298. Web.
Sadrameli, Saeed M. “Thermal/Catalytic Cracking of Hydrocarbons for the Production of Olefins: A State-Of-The-Art Review I: Thermal Cracking Review.” Fuel, vol. 140, 2015, pp. 102-115.
Salkuyeh, Yaser K, and Thomas A. Adams. “Integrated Petroleum Coke and Natural Gas Polygeneration Process.” Energy, vol. 91, 2015, pp. 479-490.
SCG Chemicals. Eco factory. 2018. SCG Chemicals Co. Web.
United States Environmental Protection Agency (EPA). “Sources of Greenhouse Gas Emissions.”EPA, Web.